

Under what condition do non-deal solutions show negative deviations?
When the new forces of interaction between the components are stronger than those in the pure components, then non-deal solutions show negative deviations.
Give an example of solution containing a solid solute in a solid solvent.
Alloy like copper dissolved in gold.
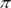 complexes?
complexes?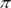 -bond with these vacant d-orbitals.
-bond with these vacant d-orbitals.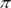 -bonded organometallic compounds.
-bonded organometallic compounds.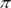 -orbitals of the ligands are called
-orbitals of the ligands are called 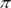 -bonded organometallic compounds.
-bonded organometallic compounds.Write the IUPAC name of the following structure.
BrCH2CH=CHCH2CHCl2
1-Bromo-5,5-dichloropent-2-ene
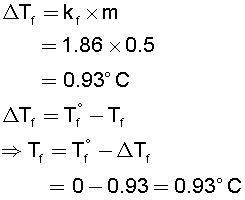
= 180
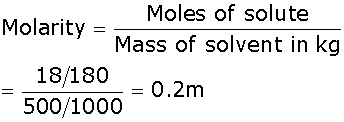
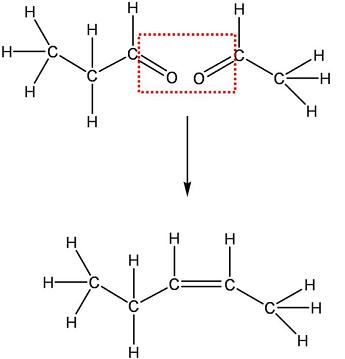
Hence, the alkene is CH3-CH2-CH=CH-CH3.
| S.No | Addition Polymers | Condensation polymers |
| 1. | They are formed by the repeated addition of molecules possessing double or triple bonds. | They are formed by repeated condensation reaction between two different bi-functional and tri-functional monomeric units. |
| 2. | E.g. polythene | E.g. nylon 66 |
Rate = k [N2O5]
k = rate/[N2O5]
k = 2.4 x 10-4/0.36
k = 6.67 x 10-4 s-1
Calculate standard free energy change for the following chemical reaction –

Explain the following observations:
(i) Sun looks red at the time of setting.
(ii) Cottrell's smoke precipitator is fitted at the mouth of the chimney used in factories.
(iii) Coagulation takes place when sodium chloride solution is added to a colloidal solution of ferric hydroxide.
(i) At the time of setting, the sun is at horizon. The light emitted by the Sun has to travel a relatively longer distance through the atmosphere. As a result, blue part of light is scattered away by the particulate in the atmosphere causing red part to be visible.
(ii) Cottrell's smoke precipitator, neutralises the charge on unburnt carbon particles, coming out of chimney and they get precipitated and settle down at the floor of the chamber.
(iii) Fe(OH)3 sol is positively charged which is coagulated by negatively charged Cl– present in sodium chloride solution.
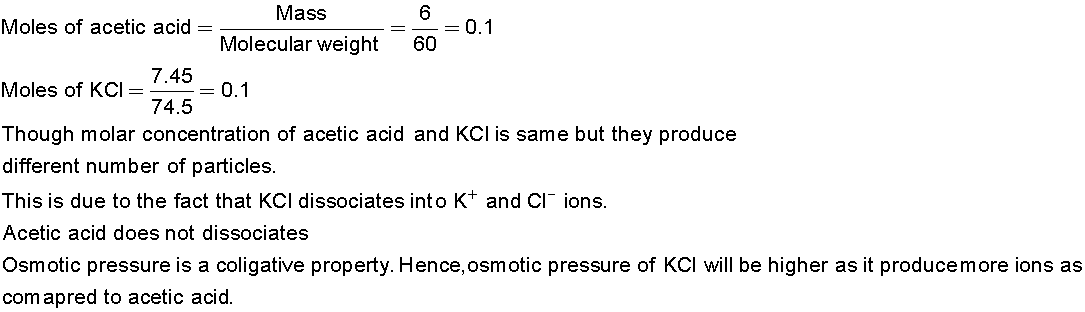
The following solutions which have higher osmotic pressure is
1. Solution having 6gL-1 of CH3COOH or
2. Solution having 7.45gL-1 of KCl
Explain.
(i) Arrange the following compounds in order of their increasing SN2 reactivity:CH3CH2Br, CH3Br, CH3Cl, (CH3)2CHCl
(ii) Arrange the following compounds in order of their increasing boiling points.
1-Bromobutane; 1,2-Dibromobutane; 2-Bromobutane; 2,3-Dibromobutane.
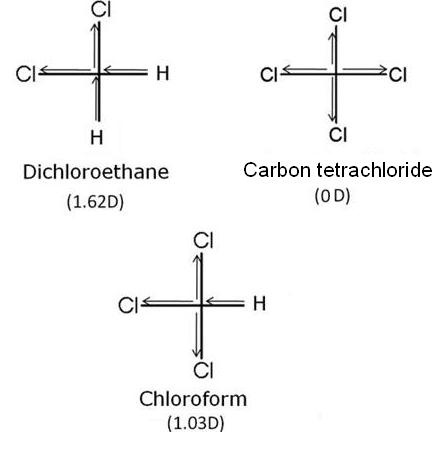
(iii) Which of the following haloalkanes have the highest dipole moment?
CHCl3, CH2Cl2 and CCl4
(i) Since SN2 reactivity decreases with increase in size of alkyl groups (-R) and C-Br bond is weaker than C-Cl bond, C-Br bond breaks easily. Therefore, the order of SN2 reactivity in the given compound will be –
(CH3)2CHCl < CH3CH2Br < CH3Cl < CH3Br
(ii) The boiling point of haloalkanes decreases with increase in branching of carbon, while increases as the number of halogen atoms increases. According to all these points, the order of boiling points of the given compounds will be given as:
(iii) Carbon tetrachloride (CCl4) has symmetrical structure, thus, it has zero dipole moments. In CHCl3 (Chloroform), the resultant of two C-Cl dipoles is opposed by the result of C-Cl and C-H bond. Thus, it has 1.03D dipole moment. In case of dichloroethane (CH2Cl2), the resultant of dipole moments of two C-Cl bond is reinforced by the resultant of dipole moments of two C-H bond. Therefore, dichloroethane has the highest dipole moment.
| | | |
|
|
|
|
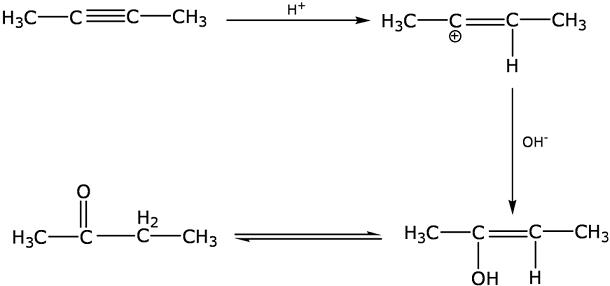
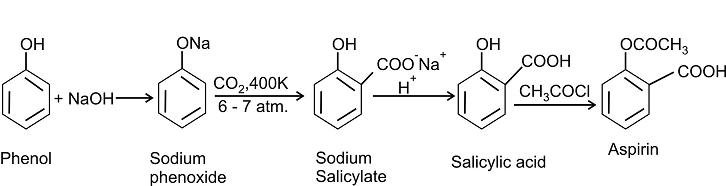
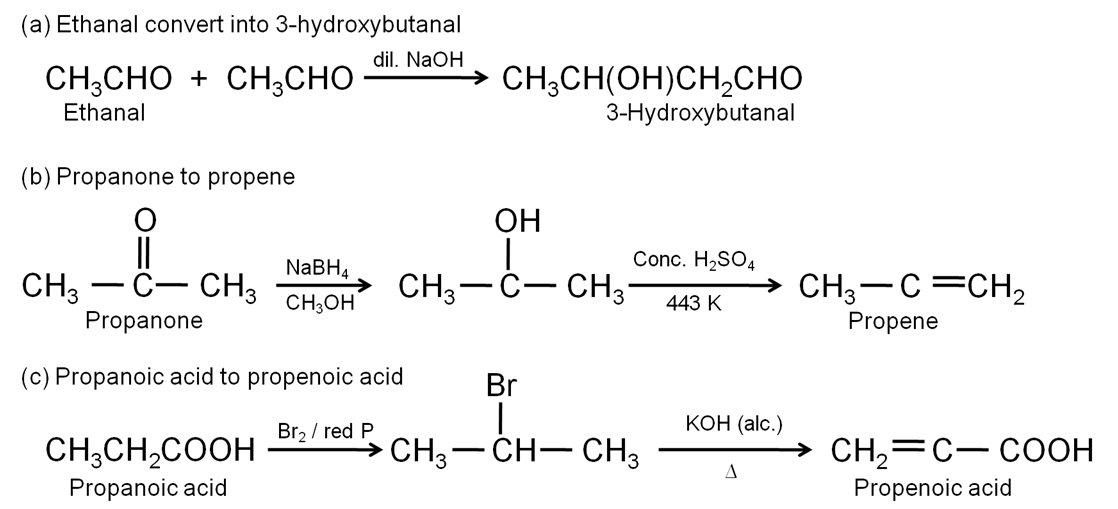
How will you carry out the following conversions?
(a) Ethanal to 3-hydroxybutanal
(b) Propanone to propene
(c) Propanoic acid to propenoic acid
c) CH3(CH2)6COO(CH2CH2O)nCH2CH2OH
hydrophobic hydrophilic
(b) CH3(CH2)15 — N(CH3)Br−
hydrophobic hydrophilic
(c) CH3(CH2)6 — COO(CH2CH2O)nCH2CH2OH
hydrophobic hydrophilic -amino acid with the carboxyl group of another molecule of the same or different
-amino acid with the carboxyl group of another molecule of the same or different  -amino acid with the elimination of a water molecule. They are classified as di-, tri-, tetra-, etc.
-amino acid with the elimination of a water molecule. They are classified as di-, tri-, tetra-, etc.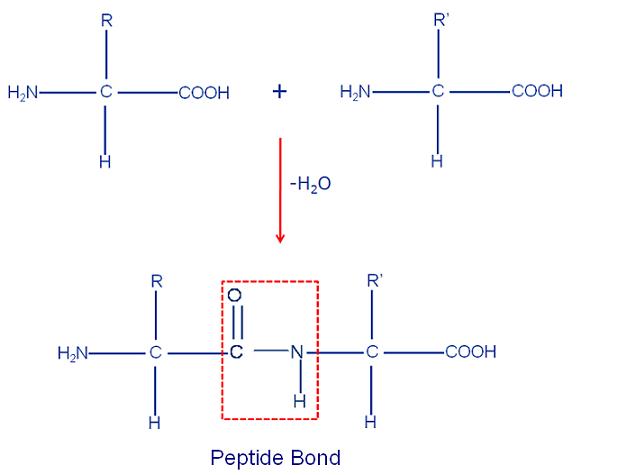
(i) Write chemical formula, chemical name and main sources of vitamin B1.
(ii) Write the deficiency disease caused by vitamin B1.
(i) Chemical name: Thiamine
Chemical formula: (C12H18N4SOCl2)
Main sources: Best source of thiamine is dried Brewer’s yeast. Also found in whole grain cereals and bread, nuts, pulses etc.
(ii) Beriberi (paralysis of legs) and loss of appetite are caused by deficiency of vitamin B1.
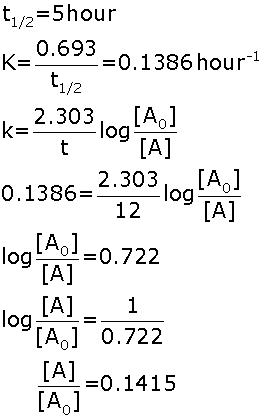
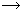 Product, is found to be first order with respect to A, second order with respect to B and zero order with respect to C.
Product, is found to be first order with respect to A, second order with respect to B and zero order with respect to C. (i) Write rate law for above reaction.
(ii) What will happen to rate of reaction when concentration of A, B and C are doubled.
i)They are molecules of large size.
ii)They have lyophobic property.
Multimolecular colloids:-
i) They are formed by the aggregation of large number of atoms or molecules which have diameter less than 1nm.
ii) They have lyophilic property.
Associated colloids:-
i) They are formed by the aggregation of large number of ions in concentrated solution
ii) They contain both lyophilic and lyophobic groups
CaCO3  CaO + CO2
CaO + CO2
 CaSiO3(slag)
CaSiO3(slag)Cu2+(aq) + H2(g) 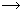 Cu(s) + 2H+(aq)
Cu(s) + 2H+(aq)
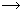 Cu(s) + Fe2+
Cu(s) + Fe2+
(a) Identify A and B in the following reactions:
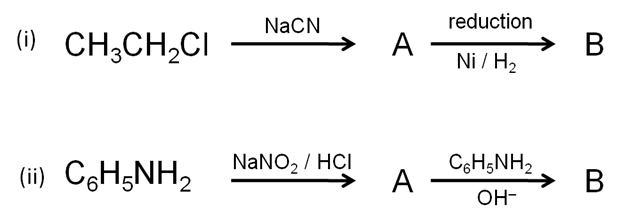
(b) pKb of aniline is more than that of methylamine.
(a)
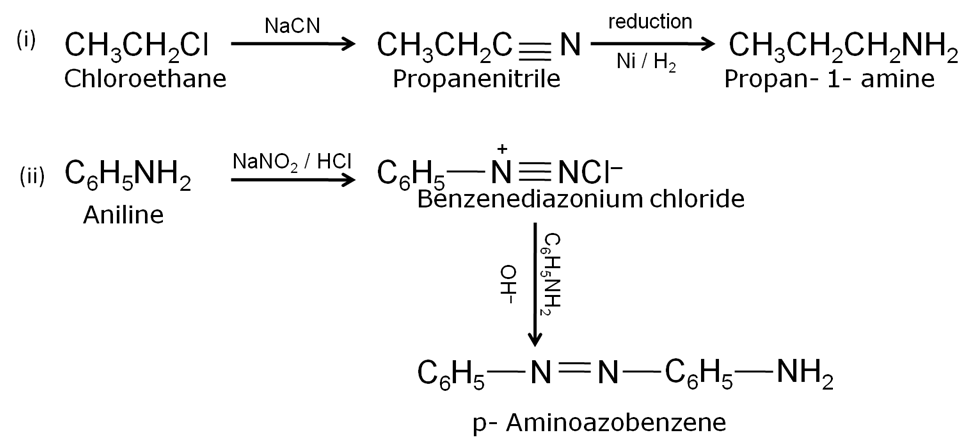
(b) In aniline, the lone pair pair of electrons on the N- atom is delocalised over the benzene ring. As a result, electron density on the nitrogen decreases. In contrast, in CH3NH2 , +I-effect of CH3 increases the electron density on the N- atom. Therefore, aniline is a weaker base than methylamine and hence its pKb value is higher than that of methylamine.
(i) Describe the orbital structure of the primary, secondary and tertiary amine.
(ii)Write IUPAC name of following:
(i) 
(ii) IUPAC name of CH3–CH2–NH–CH3 is N-methylethanamine.
IUPAC name of CH3–CH2–N(CH2CH3)–CH3 is N-ethyl-N-methylethanamine.
(i) State Faraday' first and second law of electrolysis.
(ii) If resistance of a solution is 20 ohms, what will be the conductance of the same solution?
(iii) For a weak electrolyte there is a very large increase in conductance with dilution near infinite dilution, why?
(iv) How will you calculate the dissociation constant of a weak electrolyte?
(i) Faraday' first law of electrolysis
Mass of any substance deposited or liberated at any electrode is directly proportional to the quantity of electricity passed through the electrolyte
Faraday' second law of electrolysis
When same quantity of electricity is passed through solutions of different electrolytes connected in series, the weights of the substances produced at the electrodes are directly proportional to their equivalent weights.
(ii) conductance is reciprocal of resistance, so conductance of the solution = 1/20 = 0.05 ohm-1.
(iii) For a weak electrolyte there is a very large increase in conductance with dilution near infinite dilution because as the concentration of the weak electrolyte is reduced more of it ionise.
(iv) Dissociation constant of a weak electrolyte can be calculated by the formula
Kc = c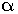 2/1 -
2/1 - 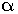 where
where  is degree of dissociation of the weak electrolyte.
is degree of dissociation of the weak electrolyte.
(i) In an electrochemical cell, salt bridge is used to maintain the electrical neutrality of the solutions in two half cells. It completes the electrical circuit by allowing the ions to flow from one solution to other without mixing the two solutions.
(ii) The absolute value of the electrode potential of a single electrode cannot be measured because oxidation and reduction half cell cannot take place alone. To overcome this difficulty, we use a reference electrode whose electrode potential is arbitrarily assigned a value.
Example: Standard or Normal Hydrogen Electrode (SHE or NHE). Its electrode potential is taken as 0.00 V at zero Kelvin.
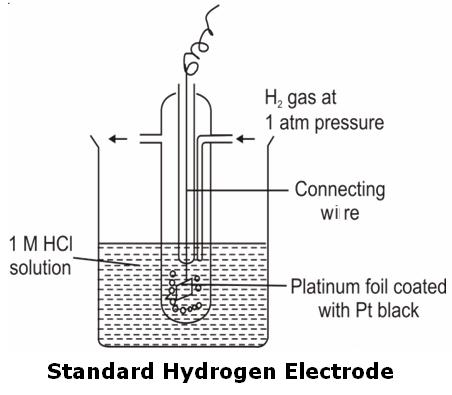
To determine the electrode potential of any electrode, a cell is set up with SHE as one of the electrodes. Since, the EMF of the SHE electrode is arbitrarily taken as 0.00 V, the EMF of the cell will directly give the electrode potential value of the other electrode.
(iii) The arrangement of various elements in order of their increasing values of electrode potentials is known as electrochemical series.
(a) Positive sign of electrode potential value represents the reduction potential. This indicates that greater the value of reduction potential, more easily the substance is reduced. It is said to be a stronger oxidising agent.
Thus, according to electrochemical series, F2 has the highest reduction potential (strongest oxidising agent) and Li+ ion has the lowest reduction potential, thus it is the weakest oxidizing agent in the series.
(b) A metal with greater oxidation potential can displace metals with lower oxidation potential from their salt solution.
For example: Decreasing order of oxidation potentials of these metals are:
Mg > Zn > Fe > Cu > Ag
Hence, each metal can displace metals on its right from the salt solutions.
(i) Due to small size, the lone pair of electrons on oxygen atoms repels the bond pair of O-O bond to a greater extent than the lone pair of electrons on the sulphur atoms in S-S bond. As a result, S-S bond (bond energy = 213 kJ/mole) is much stronger than O-O (bond energy = 138 kJ/mole) bond and hence, sulphur has much stronger tendency for catenation than oxygen. Further, as the size of atom increases down the group from sulphur to polonium, the strength of element-element bond decreases. Therefore, tendency for catenation decreases accordingly.
Example: H-S-S-S-S-H (Polysulphides)
H-O-O-H (Polyoxides)
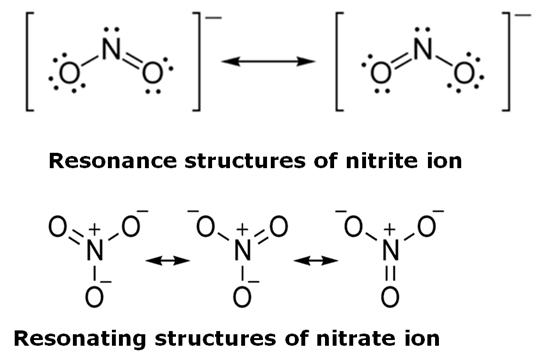
(ii) Nitrite ion shows two resonating structures. This explains that two bonds are equivalent and similar to that of double bond which has a shorter bond length.
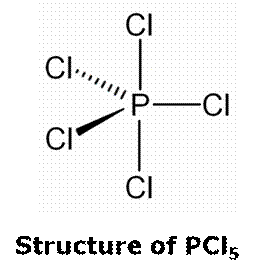
(iii) PCl5 has trigonal bipyramidal structure in both liquid and gaseous state. In this structure, two axial P-Cl bonds (219 pm) are larger than three equatorial P-Cl bonds (204 pm) because greater bond pair-bond pair repulsion exists in axial bonds. Hence, all five P-Cl bonds are not equivalent.
b) How can NH3 be obtained from NH4Cl
c) How is NH3 used for the detection of Cu2+ ions?b) 2NH4Cl + Ca(OH)2 = 2NH3 + 2H2O +CaCl2
c) N in NH3 has a lone pair of electron that it donates to the Cu2+ ions to form linkage with the metal and it leads to the formation of a deep blue colored complex which helps in the detection of Cu2+ ions.
Cu2+(aq) + 4NH3(aq) = [Cu(NH3)4]2+(aq)
(blue) (deep blue)

Take your CBSE board preparation to another level with AI based and rich media animation on Extramarks - The Learning App.
Features of Learning App