

Name the chemical which is added to a cake to make it soft and fluffy. Write its composition also.
Baking powder should be added to a cake to make it soft and fluffy. It is mixture of sodium carbonate and tartaric acid (a mild acid).
Why does dry HCl gas not turn dry blue litmus red although it is an acid?
Acids produce H+ ions only in the presence of water and thus can show acidic behaviour only in the presence of water. Therefore, dry HCl gas does not turn blue litmus paper red.
B 5.20 x 10-8
C 1.84 x 10-6
D 49 x 10-6
E 1010-1014
(a) Which of these materials is an insulator?
(b) Which of these can be used for making filaments of electric bulbs?
Why does the colour of the copper sulphate solution changes when iron nails are dipped in it?
Iron is more reactive than copper and hence it displaces copper from its salt solution (copper sulphate). Thus, colour of the copper sulphate solution changes from blue to dirty green.
-2.5 = 1/f
f = -1/2.5
f = -0.4 m
| Organism | Mode of reproduction |
| (a) Spirogyra | Fragmentation |
| (b) Plasmodium | Multiple fission |
| (c) Bread mould | Spore formation |
| (d) Planaria | Regeneration |
| Unisexual flowers | Bisexual flowers |
| A flower which contains either stamens or carpels is called a unisexual flower. For example, flowers of watermelon. | A flower which contains both stamens and carpels is called a bisexual flower. For example, flowers of mustard. |
(i)Which will undergo addition reaction- ethene or ethane?
(ii) Write IUPAC name of acetic acid.
(i)Since unsaturated hydrocarbons undergo addition reaction, ethene being unsaturated hydrocarbon will undergo addition reaction.
(ii) Ethanoic acid (Since its formula is CH3COOH).
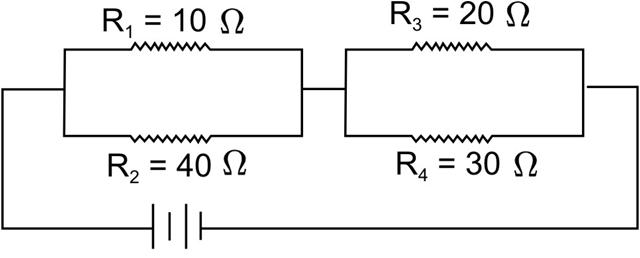
R = V/I
R = 220/5
R = 44 ohm
Number of resistors of 264 ohm that are required to make an effective resistance of 44 ohm = n
n x 1/264 = 1/44
n = 264/44
n = 6
Hence 6 resistors of 264 ohm (in parallel) are required to carry 5 A on a 220 V line.
Write balanced chemical equations for following reactions and identify the type of reaction:
(a) Zinc oxide reacts with carbon to form zinc metal and carbon monoxide.
(b) Barium chloride reacts with sodium sulphate to give barium sulphate and sodium chloride.
(a) ZnO + C  Zn + CO (Oxidation-Reduction reaction)
Zn + CO (Oxidation-Reduction reaction)
(b) BaCl2 + Na2SO4 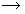 BaSO4 + 2NaCl (Double displacement reaction)
BaSO4 + 2NaCl (Double displacement reaction)
The amount of heat produced in a wire or material is given by the formula
H= I2Rt
This is known as Joule’s law of heating.
Electric heater and electric fuse are the two common instruments which work on the Joule’s law of heating.Give reasons for:
(a) While mixing an acid and water, acid must be added slowly to the water with constant stirring.
(b) Toothpastes are basic in nature.
(c) Plaster of Paris should be stored in a moisture proof container.
(a) Reaction between water and acid is a highly exothermic. During this reaction a large amount of heat is generated that can cause splashing of the mixture and burns if water is added quickly to a concentrated acid. In order to prevent this, acid is added to the water slowly with constant stirring.
(b) Bacteria present in the mouth produce acids by degrading sugar and food particles left in the mouth after eating. This can corrode teeth enamel. Thus, in order to neutralise excess acid in the mouth and prevent tooth decay, toothpastes are made basic.
(c) Plaster of Paris absorbs water molecule to form a hard solid mass of gypsum and then it cant be used for supporting fractured bons. Therefore, it should be stored in a water proof container.
Potential difference (V) = 1.5 x 3
V = 4.5 volt
Resistance (R) = 5 ohm
Current in the circuit (I) = V/R
I = 4.5/5
I = 0.9 ampere
| Acquired traits | Inherited traits |
| (i) These are the traits that are acquired by an organism during the course of its lifetime. | (i) These are the traits that are inherited by an organism from its parents. |
| (ii) These cannot be passed on to the progeny as these are not caused due to changes in the genes of the germ cells of the individual. | (ii) These can be passed on to the progeny as these are caused due to changes in the genes of the germ cells of the individual. |
(c) The zygote formed will have XY chromosomes and hence the zygote will be a male.
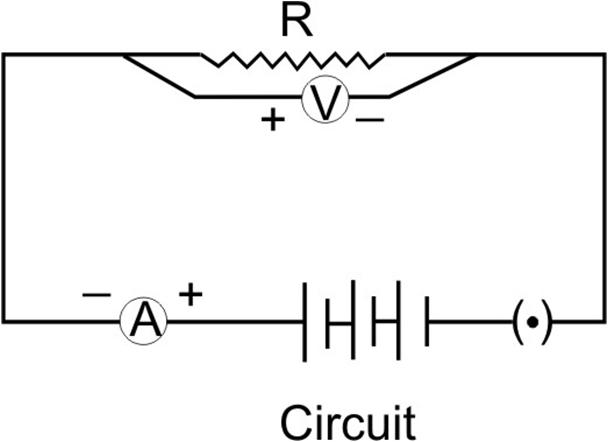
Deoxygenated blood enters the right atrium of the heart through vena cava. It is then pumped to right ventricle through atrio-ventricular aperture. It is then taken to the lungs for oxygenation via pulmonary artery. The oxygenated blood from the lungs returns back to the left atrium of the heart. It is then pumped to left ventricle which pumps it into aorta. Aorta carries the blood to different body parts.
Deoxygenated blood in right atrium ![]() Deoxygenated blood in right ventricle
Deoxygenated blood in right ventricle ![]() Deoxygenated blood in pulmonary artery
Deoxygenated blood in pulmonary artery ![]() lungs
lungs ![]() Oxygenated blood in left atrium
Oxygenated blood in left atrium ![]() Oxygenated blood in left ventricle
Oxygenated blood in left ventricle ![]() Oxygenated blood in aorta
Oxygenated blood in aorta ![]() body parts
body parts
(a) A copper coin is kept immersed in a solution of AgNO3 for some time. What will happen to
(i)The coin
(ii)Colour of the solution
(iii) Represent the change by balance chemical reaction
(b)A trivalent metal X is most abundant metal in earth’s crust.
(i) Write its name and symbol.
(ii) Write two uses of metal X.
(a) (i)Coin get dissolved in solution.
(ii)Colour of solution turn blue due to the formaIon of Cu(NO3)2
(iii) Cu + 2Ag NO3 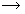 Cu (NO3)2 + 2Ag
Cu (NO3)2 + 2Ag
(b)(i)Trivalent metal could be Fe, Al, But Al is must abundant metal in earth’s crust. So metal is Aluminium (Al).
(ii)(1) Al foil is used in packaging food.
A 2,8,6
B 2,8,8
C 2,8,8,1
(ii)(ii)What will be the formula of compound between A and C.
(b) An element X burns in oxygen to form an compound XO. Write the formula of compound if this element is made to combine with chlorine and sulphur separately.
(c)What is galvanization? Why is it done?
(a) (i) Ionic
(ii) AC2
(b)Since element X forms XO with oxygen it means the valancy of element X is 2. When X is made to combine with chlorine the formula of compound will be XCl2 and with S it will give XS.
(c) It is the process of depositing a layer of Zinc over iron to prevent it fromv rusting.
(b) Light enters from air to glass having refractive index 1.65. Given that the speed of light in air is 3 x 108 m/s, what is the speed of light in the glass?
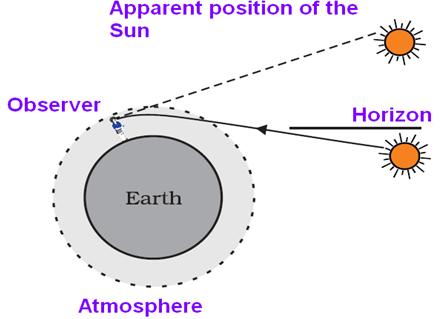
(a) 1. Sun is visible to us about 2 minutes before the actual sunrise because of atmospheric refraction.
2. The molecules of air and other fine particles in the atmosphere have size smaller than the wavelength of visible light. These are more effective in scattering light of shorter wavelengths at the blue end than light of longer wavelengths at the red end. The red light has a wavelength about 1.8 times greater than blue light. Thus, when sunlight passes through the atmosphere, the fine particles in air scatter the blue colour (shorter wavelengths) more strongly than red. The scattered blue light enters Our eyes and colour of sky appears blue.
(b).
(b) Concave mirror is used as reflector in head lights of vehicles.
(c) Convex mirror is used as rear-view mirror in automobiles.
Where will the image will be formed ,when an object is placed at the focus of a concave lens?
Image is formed between the focus and the optical centre of the concave lens.
Packets of potato chips are filled with a gas. Name this gas and explain its use.
(a)There are two test tubes having solutions A and B. The pH of solution A is 9 and pH of solution B is 5.
(i)Which of these solution has higher hydrogen ion concentrations?
(ii) Which of these solutions is acidic and which one is basic?
(a) (i) As the pH value increases the concentration of hydrogen ion decreases. Thus, solution B with pH value 5 has higher hydrogen ion concentrations.
(b) The common name of Na2CO3.10H2O is washing soda.
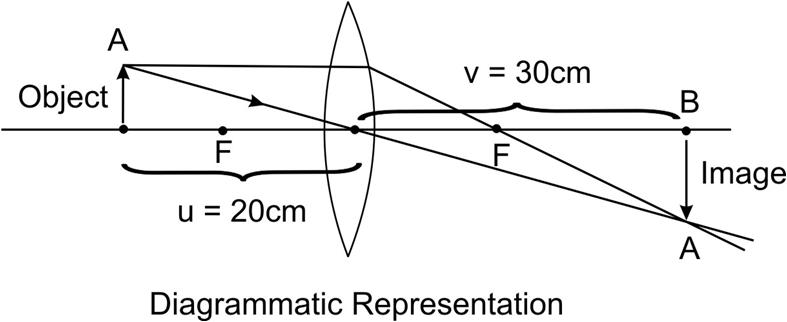
A 100 watt electric bulb is lighted for 2 hours daily and four 40 watt bulbs are lighted for 4 hours daily. Calculate the electric energy consumed in kWh in 30 days.
Energy consumed is given by the relation, E = P x T
Energy consumed by the 100 watt bulb in 30 days is given by,
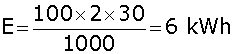
Similarly energy consumed by four 40 watt bulbs,
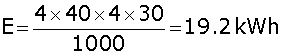
Hence, total energy consumed = 6 kWh + 19.2 kWh = 25.2 kWh.
1 2 1
(i) Yellow coloured seeds are expected in the F1 progeny as in this case yellow colour is dominant over green colour.
(ii) The self pollination of plants of F1 generation yields plants in the ratio of 3 (dominant):1 (recessive). Hence when F1 plants are self pollinated, the percentage of green coloured seeds will be 25%.
(iii) The genotypes YY and Yy will be found in the ratio 1:2.
(a) Ca(s) + 2H2O(l) → Ca(OH)2 (aq) + H2(g)
(b) K(s) + 2H2O(l) → 2KOH(aq) + H2(g) +heat
(c) Zn(s) + 2HCl (dil)→ ZnCl2(aq) + H2(g)
(a) Explain the terms
In a double displacement reaction, exchange of ions takes place among the reactants, while in displacement reaction more reactive metal displaces less reactive metal from its salt.
Example of displacement reaction:
Fe + CuSO4 Cu + FeSO4
Cu + FeSO4
Here, Fe displaces Cu from CuSO4 because Fe is more reactive than Cu.
Example of double displacement reaction:
BaCl2 (aq) + Na2SO4(aq) 2NaCl (aq) + BaSO4(s)
2NaCl (aq) + BaSO4(s)
Here, exchange of ions between reactants takes place.
An object is placed at a distance of 10cm from a concave mirror of focal length 20cm.
i. Draw a ray diagram for the formation of image.
ii. Calculate the image distance.
iii. State two characteristic of image formed.
(i)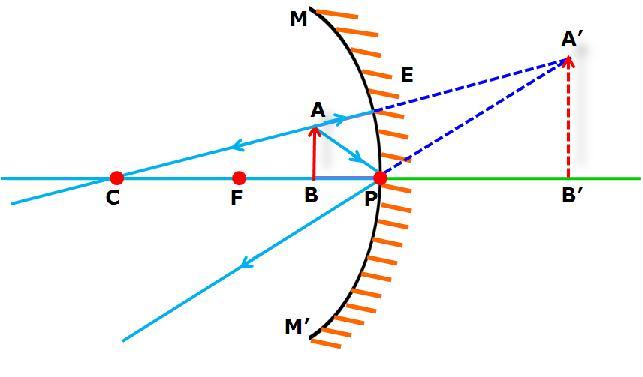
(ii) Applying mirror formula
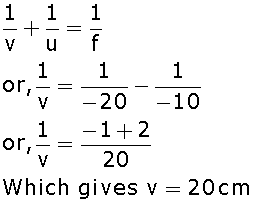
(iii) Image is virtual and erect.
Fermentation :
It is the process in which sugar molecules and starch molecules are broken down by the action of enzymes into smaller molecule ethanol and carbon dioxide.
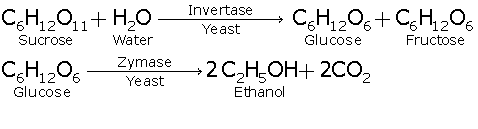
The fermentation of sugar is carried out at a controlled temperature of 20ºC to 30ºC. Fermentation produces a dilute solution of ethanol in water. Ethanol is separated from water and purified by the process of distillation.
Uses :
1. Ethanol is used for making antifreeze mixtures.
2. Ethanol is used as fuel in internal combustion engine and in spirit lamps.
Harmful effects of drinking alcohol :
a. Ethanol is harmful for lungs and causes addiction.
b. Ethanol can create heart problems.
c. It effects on the nervous system.
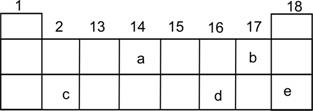
(i)Select the letter which represents a halogen.
(ii)Select the letter which represents a noble gas.
(iii)What type of bond is formed between a and b?
(iv)What type of bond is formed between c and d?
(v)Which element will form a divalent anion?
A student ads a spoon full of powdered sodium hydrogen carbonate to a flask containing ethanoic acid. List two main observations, he must note in his note book, about the reaction that takes place. Also write chemical equation for the reaction.
The student must note the following observations:
(a) Immediate fizzing occurred on adding sodium hydrogen carbonate to ethanoic acid.
(b) A colourless gas (carbon dioxide) evolved due to the reaction of sodium hydrogen carbonate to ethanoic acid. At the end of the reaction, a colourless solution obtained.
A student is observing a permanent slide showing sequentially the different stages of asexual reproduction taking place in yeast. Name this process and draw diagrams, of what he observes, in a proper sequence.
The process is budding.
What do you observe when you drop a few drops of acetic acid to atest tube containing:
(i) phenolphthalein
(ii) distilled water
(iii) universal indicator
(iv) sodium hydrogen carbonate powder
(i) No change in colour is observed.
(ii) No change in colour is observed.
(iii) The colour of solution turns orange.
(iv) Evolution of a colourless and odorless gas with brisk effervescence is observed.
A student focuses the image of a well illuminated distant object on a screen using a convex lens. After that he gradually moves the object towards the lens and each time focuses its image on the screen by adjusting the lens.
(i) In which direction-towards the screen or away from the screen, does he move the lens?
(ii) What happens to the size of the image-does it decrease or increase?
(iii) What happens to the image on the screen when he moves the object very close to the lens?
(i)As the distance between the object and the lens decreases, the distance between the lens and the image increases. Therefore, in order to obtain the image on the screen, he has to move the lens away from the screen.
(ii) In case of a convex lens, the size of the image increases as the distance between the object and the lens decreases.
(iii) When an object is placed between the focus and the optical centre of a convex lens, the image formed is virtual and erect. A virtual image is not obtained on the screen. Thus, when the object is placed very near to the convex lens, it will disappear from the screen.
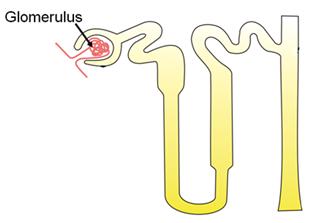
Draw a labelled diagram to show a particular stage of binary fission in Amoeba in which, its nucleus elongates and divides into two and a constriction appears in its cell membrane.
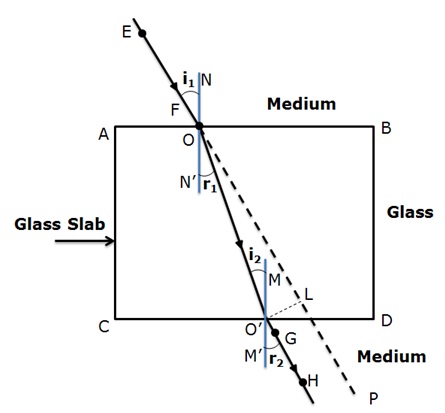
“A ray of light incident on a rectangular glass slab immersed in any medium emerges parallel to itself.” Draw labelled ray diagram to justify the statement.
Below given is the ray diagram in which the ray EF is the incident ray and GH is the emergent ray which is parallel to the incident ray.

Take your CBSE board preparation to another level with AI based and rich media animation on Extramarks - The Learning App.
Features of Learning App