Chemistry:2019:CBSE:[Delhi]: Set-II
To Access the full content, Please Purchase
-
Q1
Why are medicines more effective in colloidal state?
Marks:1View AnswerAnswer:
Medicines are more effective in colloidal state because they have large surface area in colloidal form. Therefore, they are easily assimilated and absorbed by the body tissues.
-
Q2
What is difference between an emulsion and a gel?
Marks:1View AnswerAnswer:
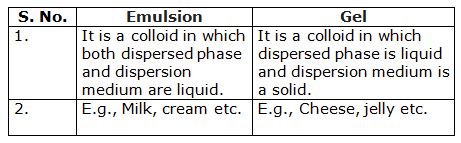
-
Q3
Arrange the following in increasing order of base strength in gas phase.
(C2H5)3N, C2H5NH2, (C2H5)2NH
Marks:1View AnswerAnswer:
There is no solvation effect in gas phase. Therefore, the basic strength depends upon the +I effect. The higher the +I effect, the stronger is the base. Greater the number of alkyl groups attached with nitrogen, higher is the +I effect. Thus, the given compounds can be arranged in the increasing order of their base strength in the gas phase as follows.
C2H5NH2 < (C2H5)2NH < (C2H5)3N
-
Q4
Why conductivity of silicon increases on doping with phosphorus?
Marks:1View AnswerAnswer:
Conductivity of silicon increases on doping with phosphorus because phosphorus atoms in silicon crystal cause n-type doping. Phosphorus atoms have 5 valence electrons. Four of these valence electrons form covalent bonds with neighbouring silicon atoms and the fifth valence electron is free and gets delocalised. These delocalised electrons increase the conductivity of silicon.
-
Q5
What is the basic structural difference between glucose and fructose?
Marks:1View AnswerAnswer:
Glucose has an aldehyde group and fructose has ketone group. Glucose is an aldohexose while, ketone is a ketohexose.
-
Q6
Write the products obtained after hydrolysis of lactose.
Marks:1View AnswerAnswer:
The products obtained after hydrolysis of lactose are β-D-Glucose and β-D-Galactose.
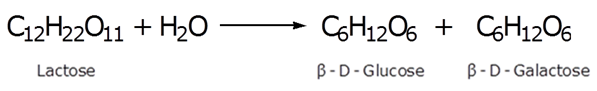
-
Q7
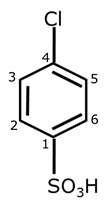
Write IUPAC name of the given compound.
 Marks:1View Answer
Marks:1View AnswerAnswer:
4-Chlorobenzenesulphonic acid
-
Q8
Write structures of compounds A and B in each of the following reactions:
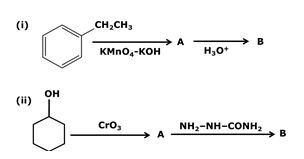 Marks:2View Answer
Marks:2View AnswerAnswer:
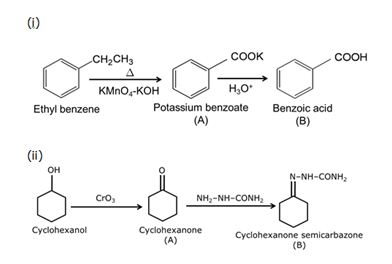
-
Q9
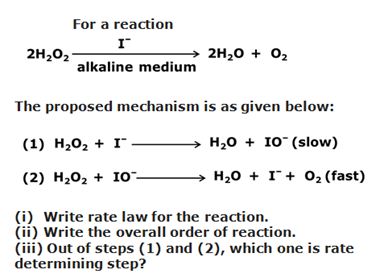 Marks:2View Answer
Marks:2View AnswerAnswer:
(i) For a complex reaction, the rate of reaction is expressed by the slowest step. Since, in the given reaction, step (1) is the slow step, the expression of rate law for the reaction is written as follows:
Rate = K [H2O2][I–]
(ii) Order of the reaction is the sum of the exponents to which the concentration terms are raised in the rate law expression.
Hence, the order of the reaction= 1+1=2
(iii) In a multiple step reaction, the slowest step is the rate determining step, therefore, out of steps (1) and (2), step (1) is the rate determining step.
-
Q10
Write two differences between an ideal and a non-ideal solution.
Marks:2View AnswerAnswer:
Two differences between an ideal and a non-ideal solution are as follows:
S. No.
Ideal solution
Non-ideal solution
1.
It is a solution in which each component of the solution obeys Raoult’s law at all temperatures and concentrations.
It is a solution in which each component of the solution does not obey Raoult’s law at all temperatures and concentrations.
2.
There is no enthalpy change when pure components are mixed, i.e., ΔH = 0.
There is a definite enthalpy change when pure components are mixed, i.e., ΔH ≠ 0.



