Chemistry:2019:CBSE:[Delhi]: Set-I
To Access the full content, Please Purchase
-
Q1
Out of NaCl and AgCl, which one shows Frenkel defect and why?
Marks:1View AnswerAnswer:
Frenkel defect arises when smaller ion (usually cation) is missing from its lattice site and occupies the interstitial site. It occurs in compounds with low coordination number and large difference in the size of cations and anions.
The difference in the sizes of Ag+ and Cl– ions is large and the difference in the sizes of Na+ and Cl– ion is small. In case of AgCl, the smaller Ag+ ion (as compared to Cl– ion) easily moves in interstitial space. Therefore, out of NaCl and AgCl, AgCl will show Frenkel defect.
-
Q2
Arrange the following in increasing order of boiling points:
(CH3)3N, C2H5OH, C2H5NH2
Marks:1View AnswerAnswer:
The increasing order of boiling points is
(CH3)3N< C2H5NH2< C2H5OH
Intermolecular hydrogen bonding affects the boiling points.
(CH3)3N : Does not form intermolecular hydrogen bonds as it does not have hydrogen atom bonded to nitrogen atom.
C2H5NH2 : Forms intermolecular hydrogen bonds due to the presence of hydrogen atom bonded to nitrogen atom.
C2H5OH : The extent of intermolecular hydrogen bonding and strength of hydrogen bonds is more in C2H5OH as compared to C2H5NH2 due to higher electronegativity of oxygen as compared to nitrogen.
-
Q3
Why are medicines more effective in colloidal state?
Marks:1View AnswerAnswer:
Medicines are more effective in colloidal state because they have large surface area in colloidal form. Therefore, they are easily assimilated and absorbed by the body tissues.
-
Q4
What is difference between an emulsion and a gel?
Marks:1View AnswerAnswer:
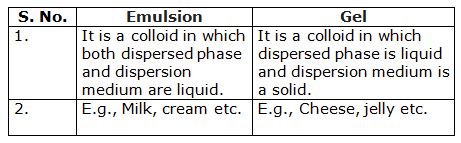
-
Q5
Define ambident nucleophile with an example.
Marks:1View AnswerAnswer:
Ambident nucleophile is a nucleophile that can attack or link from two or more sites resulting in two or more products. It has two or more nucleophilic centres. For example, nitrite ion has two different points for linkage.
-
Q6
What is the basic structural difference between glucose and fructose?
Marks:1View AnswerAnswer:
Glucose has an aldehyde group and fructose has ketone group. Glucose is an aldohexose while, ketone is a ketohexose.
-
Q7
Write the products obtained after hydrolysis of lactose.
Marks:1View AnswerAnswer:
The products obtained after hydrolysis of lactose are β-D-Glucose and β-D-Galactose.
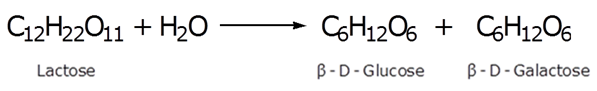
-
Q8
Write balanced chemical equations for the following processes:
(1) XeF2 undergoes hydrolysis.
(2) MnO2 is heated with conc. HCl.
Marks:2View AnswerAnswer:
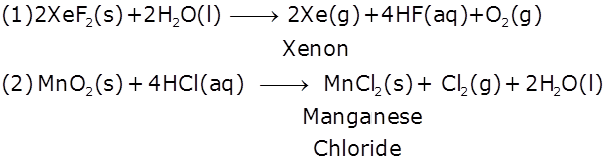
-
Q9
Arrange the following in order of property indicated for each set:
(1) H2O, H2S, H2Se, H2Te - increasing acidic character
(2) HF, HCl, HBr, HI - decreasing bond enthalpy
Marks:2View AnswerAnswer:
(1)Acidic character of hydrides of group 16 elements increases down the group, from H2O to H2Te due to the decrease in bond dissociation enthalpy of H–E bond, (where E=O, S, Se, Te) down the group.
H2O< H2S< H2Se< H2Te
(2) HF > HCl > HBr > HI
The stability of the halides of group 17 elements (HX, where X= halogen) decreases down the group due to the decrease in bond (H–X) dissociation enthalpy.
-
Q10
State Raoult's law for a solution containing volatile components. Write two characteristics of the solution which obeys Raoult's law at all concentrations.
Marks:2View AnswerAnswer:
Raoult's law for a solution containing volatile components states that the partial vapour pressure of each component in the solution is directly proportional to its mole fraction present in solution.
Characteristics of solution which obey Raoult's law at all concentrations are as follows:
(i) The change in volume on mixing is zero.
(ii) The change in enthalpy on mixing is zero.



