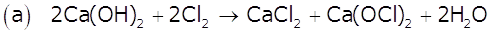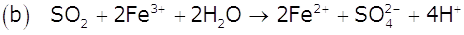Chemistry:2019:CBSE:[All India]: Set-II
To Access the full content, Please Purchase
-
Q1
What is the basic structural difference between starch and cellulose?
Marks:1View AnswerAnswer:
Starch is a polymer of α- glucose whereas cellulose is a polymer of β- glucose.
-
Q2
Write the products obtained after hydrolysis of DNA.
Marks:1View AnswerAnswer:
The products of hydrolysis of DNA are as follows:
2-deoxyribose + nitrogen containing heterocyclic base + phosphoric acid
-
Q3
Arrange the following in increasing order of pKb values:
C6H5CH2NH2, C6H5NHCH3, C6H5NH2
Marks:1View AnswerAnswer:
C6H5CH2NH2 < C6H5NHCH3 < C6H5NH2
-
Q4
What type of colloid is formed when a solid is dispersed in a liquid? Give an example.
Marks:1View AnswerAnswer:
Sol is formed when a solid is dispersed in a liquid. Paint is an example of sol.
-
Q5
Out of Chlorobenzene and Cyclohexyl chloride, which one is more reactive towards nucleophilic substitution reaction and why?
Marks:1View AnswerAnswer:
Out of Chlorobenzene and Cyclohexyl chloride, Cyclohexyl chloride is more reactive towards nucleophilic substitution reaction due to partial double bond character of C-Cl bond in Chlorobenzene which is difficult to break. Also chlorobenzene is resonance stablised.
-
Q6
Out of KCl and AgCl, which one shows Schottky defect and why?
Marks:1View AnswerAnswer:
Schottky defect is shown by ionic compounds having small difference in the size of cations and anions. Out of KCl and AgCl, it is shown by KCl, due to comparable sizes of K+ and Cl– ions.
-
Q7
Why does ZnO appear yellow on heating?
Marks:1View AnswerAnswer:
When zinc oxide is heated, it loses oxygen and there is an excess of Zn2+ ions. The number of excess Zn2+ ions trapped in the interstitial sites is equal to the number of electrons trapped in neighbouring interstitial sites. The presence of free electrons gives rise to the enhanced electrical conductivity and yellow colour to the crystal.
-
Q8
When FeCr2O4 is fused with Na2CO3 in the presence of air it gives a yellow solution of compound (A). Compound (A) on acidification gives compound (B). Compound (B) on reaction with KCl forms an orange coloured compound (C). An acidified solution of compound (C) oxidises Na2SO3 to (D). Identify (A), (B), (C) and (D).
Marks:2View AnswerAnswer:
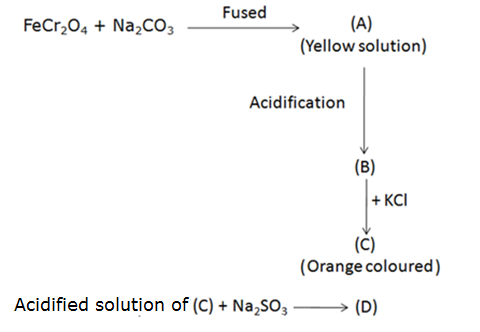
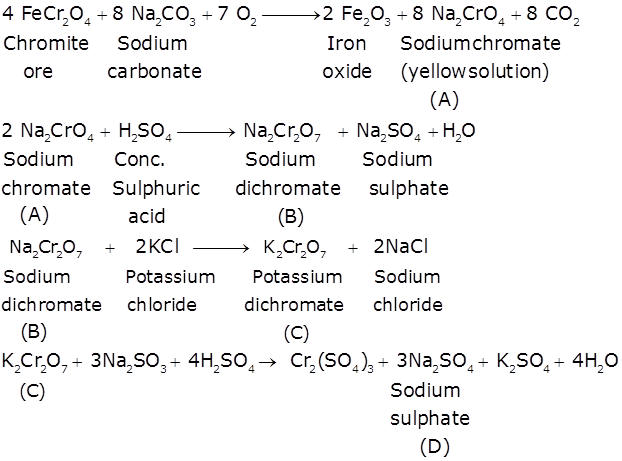
-
Q9
Give reasons:
(a) A decrease in temperature is observed on mixing ethanol and acetone.
(b) Potassium chloride solution freezes at a lower temperature than water.
Marks:2View AnswerAnswer:
(a) Interaction between ethanol and acetone is weaker than the interaction between pure ethanol or acetone. Therefore, a decrease is observed on mixing ethanol and acetone.
(b) On adding non-volatile solute like KCl to water, the vapour pressure of solution decreases. Therefore, potassium chloride solution freezes at a lower temperature than water.
-
Q10
Write balanced chemical equations for the following processes:
(a) Cl2 is passed through slaked lime.
(b) SO2 gas is passed through an aqueous solution of Fe(III) salt.
Marks:2View AnswerAnswer: