Chemistry:2016:CBSE:[Delhi]:Set-III
To Access the full content, Please Purchase
-
Q1
What type of magnetism is shown by a substance if magnetic moments of domains are arranged in same direction?
Marks:1View AnswerAnswer:
Ferromagnetism is shown by the substances in which the magnetic moments of domains are arranged in the same direction.
-
Q2
which is more reactive towards SN1 reaction and why?Marks:1View AnswerAnswer:
In the SN1 reaction formation of carbocation is the rate-determining step and the stability of carbocation determines the reactivity (rate of reaction)
The order of stability of carbocation is as follows:
Tertiary > Secondary > Primary
1-chloro-1-methylpropane forms secondary carbocation, while 1-chloro-2-methylpropane forms primary carbocation. Since the secondary carbocation is more stable than primary carbocation, the reactivity of 1-chloro-1-methylpropane will be more for the SN1 reaction.
-
Q3
On adding NaOH to ammonium sulphate, a colourless gas with pungent odour is evolved, which forms a blue-coloured complex with Cu2+ ion. Identify the gas.
Marks:1View AnswerAnswer:
When NaOH is added to ammonium sulphate, ammonia is evolved. It has a characteristic pungent odour and forms a blue-coloured complex with the Cu2+ ion.
-
Q4
Write the main reason for the stability of colloidal sols.
Marks:1View AnswerAnswer:
The presence of equal and similar charges on colloidal particles and Brownian movement are largely responsible for the stability to the colloidal solution.
-
Q5
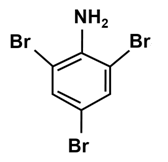
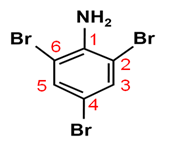
Write the IUPAC name of the given compound:
 Marks:1View Answer
Marks:1View AnswerAnswer:
The IUPAC name of the given compound is 2, 4, 6-tribromoaniline.
-
Q6
When a coordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex.
(ii) IUPAC name of the complex.
Marks:2View AnswerAnswer:
(i) Formation of two moles of AgCl indicates that in the structural formula of the coordination compound, two Cl− ions are satisfying the primary valency while five H2O molecules and one Cl− ion are present inside the coordination sphere. It means that the coordination number of Cr is 6 and one H2O molecule is present as the water of hydration.
Structural formula: [Cr(H2O)5Cl]Cl2.H2O
(ii)IUPAC name of the compound: Pentaaquachloridochromium(III) chloride
-
Q7
From the given cells:
Lead storage cell, Mercury cell, Fuel cell and Dry cell
Answer the following:
(i) Which cell is used in hearing aids?
(ii) Which cell was used in Apollo Space Programme?
(iii) Which cell is used in automobiles and inverters?
(iv) Which cell does not have long life?
Marks:2View AnswerAnswer:
(i) Mercury cell is used in hearing aids.
(ii) Fuel cell was used in the Apollo space programme.
(iii) Lead storage cell is used in automobiles and inverters.
(iv) Dry cell does not have a long life.
-
Q8
When chromite ore, FeCr2O4 is fused with NaOH in presence of air, a yellow-coloured compound (A) is obtained, which on acidification with dilute sulphuric acid gives a compound (B). Compound (B) on reaction with KCl forms an orange coloured crystalline compound (C).
(i) Write the formulae of the compounds (A), (B) and C.
(ii) Write one use of compound (C).
Marks:2View AnswerAnswer:
(i)
On acidification, sodium chromate gives sodium dichromate (B).
Therefore, compound (B) is sodium carbonate (Na2Cr2O7).
Sodium dichromate, on reaction with KCl, forms potassium dichromate (orange-coloured crystalline compound).
Therefore, compound (C) is potassium dichromate (K2Cr2O7).
(ii) Potassium dichromate is used in preparation of azo compounds.
-
Q9
Complete the following chemical equations:
Marks:2View AnswerAnswer:
-
Q10
Write the mechanism of the following reaction:
Marks:2View AnswerAnswer:
The mechanism of the given reaction is as follows:



