Chemistry:2014 :CBSE [ Delhi]: Set- III
To Access the full content, Please Purchase
-
Q1
Give one example each of lyophobic sol and lyophilic sol.
Marks:1View AnswerAnswer:
(i) Lyophilic sol: Gelatin
(ii) Lyophobic sol: Iron (III) hydroxide solution [Fe(OH)3]
-
Q2
Write the IUPAC name of the compound.
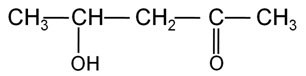 Marks:1View Answer
Marks:1View AnswerAnswer:
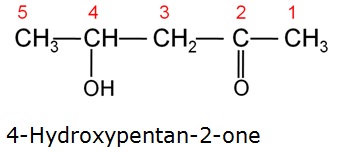
-
Q3Marks:1View Answer
Answer:
Dipole-dipole interaction exists between methanol and acetone (because both are polar molecules).
-
Q4
Which of the following is a more stable complex and why?

Marks:1View AnswerAnswer:
The complexes having chelating ligands are more stable as compared to those which do not have chelating ligands. Since ethylene diammine(en) is a bidentate ligand and forms stable chelate,
therefore, [Co(en)3]3+ is a more stable complex than [Co(NH3)6]3+. -
Q5
Arrange the following in increasing order of basic strength:
C6H5NH2, C6H5NHCH3, C6H5N(CH3)2Marks:1View AnswerAnswer:
Increasing order of basic strength is as follows:
C6H5NH2 < C6H5NHCH3 < C6H5N(CH3)2 -
Q6
Name the products of hydrolysis of sucrose.
Marks:1View AnswerAnswer:
Products of hydrolysis of sucrose are D-(+) glucose and D-(-)-fructose.
-
Q7
Which of the following isomers is more volatile: o-nitrophenol or p-nitrophenol?
Marks:1View AnswerAnswer:
o-nitrophenol is more volatile than p-nitrophenol.
-
Q8
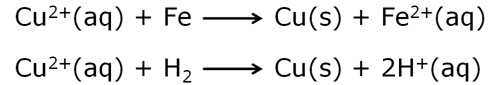
Which reducing agent is employed to get copper from the leached low-grade copper ore?
Marks:1View AnswerAnswer:
Scrap iron or hydrogen is used as reducing agent to obtain copper from the leached low-grade copper ore.
-
Q9
State Raoult's law for the solution containing volatile components. What is the similarity between Raoult's law and Henry's law?
Marks:2View AnswerAnswer:
Raoult's law states that for a solution of volatile liquids, the partial vapour pressure of each component in the solution is directly proportional to its mole fraction.
Thus, if there is a solution of two liquid components (1 and 2), then for component 1:

-
Q10Marks:2View Answer
Answer:
(i) Rate Constant
Rate constant is defined as the rate of a reaction when concentration of the reactants is unity.
Unit of k is mol1−nLn−1s−1, where n is the order of the reaction.
(ii) Half life period of a reaction (t1/2)
The half life of a reaction is the time period in which the concentration of a reactant is reduced to one half of its initial concentration.



