Chemistry:2014: CBSE [ All India] :Set-I
To Access the full content, Please Purchase
-
Q1
Why is adsorption always exothermic?
Marks:1View AnswerAnswer:
Adsorption is a surface phenomenon.Adsorbate molecules are held to adsorbent due to attractive interactions. Since energy is always released during attractive interactions, adsorption is always exothermic.
-
Q2
Name the method that is used for refining of nickel.
Marks:1View AnswerAnswer:
Mond process is used in refining of nickel.
-
Q3
Why does NO2 dimerise?
Marks:1View AnswerAnswer:
NO2 contains odd number of valence electrons. It dimerises, so that it can have even number of electrons in valence shell and form a more stable N2O4 molecule.
-
Q4
Based on molecular forces, what type of polymer is neoprene?
Marks:1View AnswerAnswer:
Neoprene is an elastomer.
-
Q5
What are the products of hydrolysis of maltose?
Marks:1View AnswerAnswer:
Maltose gives two units of
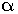 -D-glucose on hydrolysis.
-D-glucose on hydrolysis. -
Q6
Write the structure of 4-chloropentan-2-one.
Marks:1View AnswerAnswer:
4-Chloropentan-2-one has the following structure:
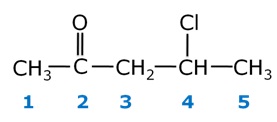
-
Q7
Identify the chiral molecule in the following pair:
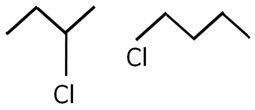 Marks:1View Answer
Marks:1View AnswerAnswer:
A chiral molecule is the one which has a carbon atom bearing four different groups. In the following pair of molecules, the chiral molecule is:
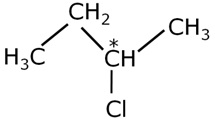
-
Q8
The conversion of primary aromatic amines into diazonium salts is known as __________ .
Marks:1View AnswerAnswer:
The conversion of primary aromatic amines into diazonium salts is known as diazotisation.
-
Q9
Write the names of monomers used for getting the following polymers:
(i) Terylene
(ii) Nylon-6,6Marks:2View AnswerAnswer:
(i) The monomers of terylene are ethylene glycol and terephthalic acid.
(ii) The monomers of nylon-6,6 are hexamethylenediamine and adipic acid. -
Q10
Describe the roles of the following:
(i) SiO2 in the extraction of copper from copper matte
(ii) NaCN in froth floatation processMarks:2View AnswerAnswer:
(i) In the extraction of copper from copper matte, SiO2 is used to remove iron oxide as slag.
FeO + SiO2
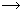 FeSiO3
FeSiO3(ii) In the froth floatation process, NaCN is used as a depressant. During separation of two sulphide ores, it selectively prevents one ore from forming froth. For example, when NaCN is added to ores containing ZnS and Pbs, it allows PbS to come with froth, but prevents ZnS from coming to froth by forming Na2[Zn(CN)4] on reaction with ZnS.



