Chemistry:2013:CBSE: [Delhi]:Set-III
To Access the full content, Please Purchase
-
Q1
What types of substances would make better permanent magnets, ferromagnetic or ferrimagnetic?
Marks:1View AnswerAnswer:
Ferromagnetic substances would make better permanent magnets. The reason for such a magnetic behaviour by the ferromagnetic substances is the parallel alignment of the magnetic moments of domains.
-
Q2
What is the composition of ‘copper matte’?
Marks:1View AnswerAnswer:
Copper matte chiefly consists of Cu2S along with some unchanged FeS.
-
Q3
What is a glycosidic linkage?
Marks:1View AnswerAnswer:
Two monosaccharide units are joined together through an ethereal or oxide linkage formed by the loss of a water molecule, this linkage is called the glycosidic linkage.
-
Q4
Write the IUPAC name of (CH3)2CHCH(Cl)CH3.
Marks:1View AnswerAnswer:
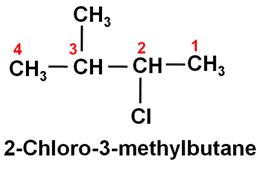
-
Q5
Which compound in the following pair undergoes faster SN1 reaction?
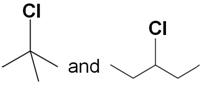 Marks:1View Answer
Marks:1View AnswerAnswer:
The reactivity in the SN1 reaction is governed by the stability of the intermediate carbocation, which an alkyl halide gives on ionisation.
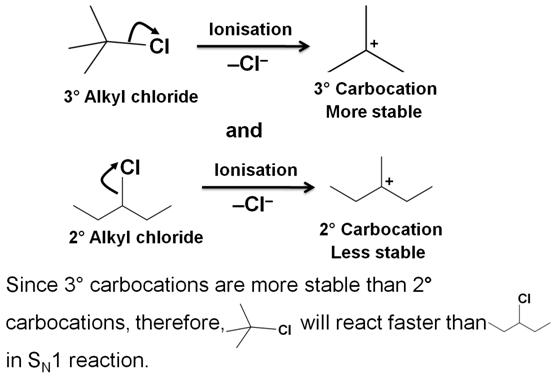
-
Q6
Write the structure of p-methylbenzaldehyde molecule.
Marks:1View AnswerAnswer:
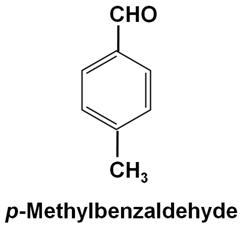
-
Q7
What is the covalency of nitrogen in N2O5?
Marks:1View AnswerAnswer:
The covalency of the nitrogen depends upon the number of shared pair of electrons. In N2O5, each nitrogen atom has four shared pair of electrons. Therefore, the covalency of the nitrogen in N2O5 is 4.
-
Q8
Arrange the following in the increasing order of their basic strength in the aqueous solution:
CH3NH2, (CH3)3N, (CH3)2NHMarks:1View AnswerAnswer:
The increasing order of the basic strength of methylamines in the aqueous solution is as follows:
(CH3)3N < CH3NH2 < (CH3)2NH -
Q9
How will you convert the following:
(i) Propene to propan-1-ol
(ii) Ethanal to propan-2-olMarks:2View AnswerAnswer:
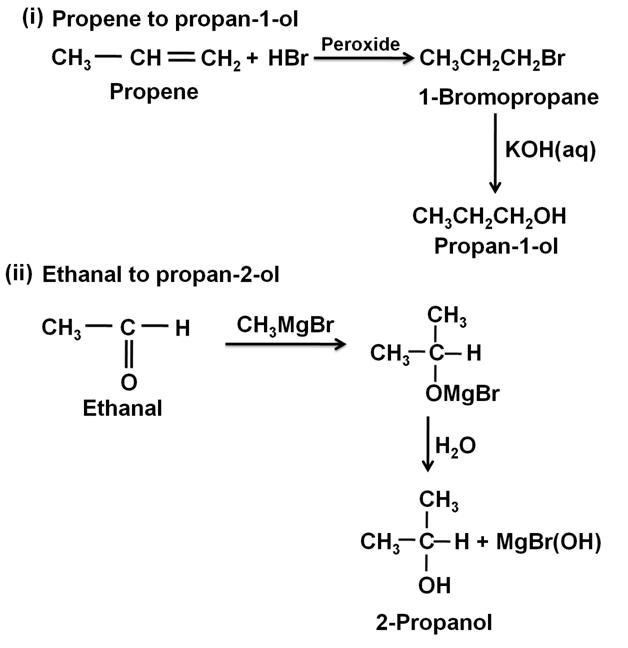
-
Q10
What is the difference between multimolecular and macromolecular colloids? Give one example of each.
Marks:2View AnswerAnswer:
Multimolecular colloids
Macromolecular colloids
1. They are formed by the aggregation of a large number of atoms or molecules, which generally have diameter less than 1 nm. For example, gold sol, sulphur sol, etc.
1. They are the colloid molecules, which have very high molecular masses. For example, rubber, cellulose, etc.
2. Their atoms or molecules are held together by weak van der Waals forces.
2. Due to the presence of the long chains, the van der Waals forces holding them are comparatively stronger.



