Chemistry:2013:CBSE:[Delhi]:Set-II
To Access the full content, Please Purchase
-
Q1
Name the method used for the refining of the nickel metal.
Marks:1View AnswerAnswer:
Mond’s process is used for refining of nickel.
-
Q2
Arrange the following in the increasing order of their basic strength in the aqueous solution:
CH3NH2, (CH3)3N, (CH3)2NHMarks:1View AnswerAnswer:
The increasing order of the basic strength of methylamines in the aqueous solution is as follows:
(CH3)3N < CH3NH2 < (CH3)2NH -
Q3
What type of stoichiometric defect is shown by AgCl?
Marks:1View AnswerAnswer:
The stoichiometric defect that is shown by AgCl is the Frenkel defect.
-
Q4
Write the IUPAC name of:
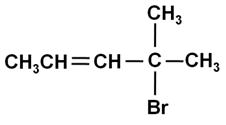 Marks:1View Answer
Marks:1View AnswerAnswer:
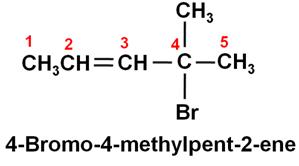
-
Q5
What types of bonding helps in stabilising the
 –helix structure of proteins?Marks:1View Answer
–helix structure of proteins?Marks:1View AnswerAnswer:
The
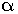 –helix structure of the proteins is stabilised by the intramolecular H-bonding between >C=O of one amino acid residue and the N–H of the fourth amino acid residue in the chain.
–helix structure of the proteins is stabilised by the intramolecular H-bonding between >C=O of one amino acid residue and the N–H of the fourth amino acid residue in the chain. -
Q6
What inspired N. Bartlett for carrying out the reaction between Xe and PtF6?
Marks:1View AnswerAnswer:
N. Bartlett observed that PtF6 reacts with O2 to yield an ionic solid, O2+PtF6–.
In this reaction, O2 gets oxidised to O2+ by PtF6.
He thought, since the first ionisation enthalpy of Xe is fairly close to that of the O2 molecule, hence PtF6 should also be oxidised Xe to Xe+. This inspired N. Bartlett for carrying out the reaction between Xe and PtF6. -
Q7
What happens when ethyl chloride is treated with the aqueous KOH?
Marks:1View AnswerAnswer:
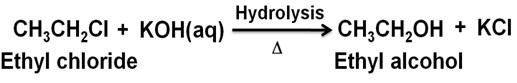
-
Q8
Write the structure of 4-chloropentan-2-one.
Marks:1View AnswerAnswer:
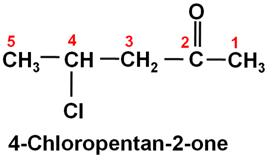
-
Q9
18 g of glucose, C6H12O6 (Molar Mass = 180 g mol–1) is dissolved in 1 kg of water in a sauce pan. At what temperature will this solution boil?
(Kb for water = 0.52 K kg mol–1, boiling point of pure water = 373.15 K)Marks:2View AnswerAnswer:
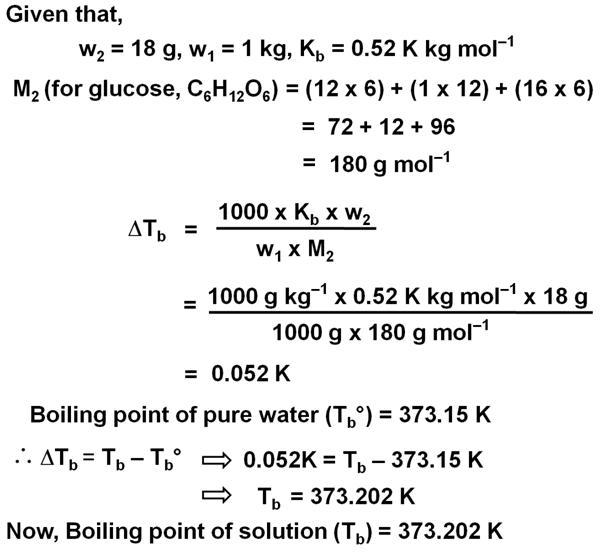
-
Q10
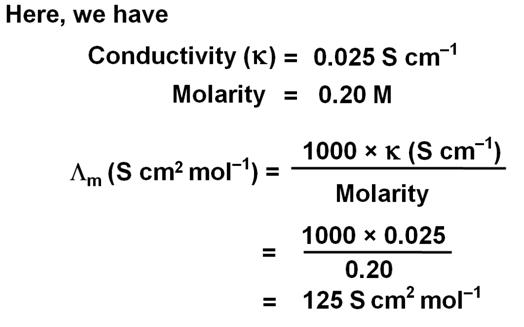
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm–1. Calculate its molar conductivity.
Marks:2View AnswerAnswer: