Chemistry:2013:CBSE:[ All India]:Set-II
To Access the full content, Please Purchase
-
Q1
Of physisorption or chemisorption, which has a higher enthalpy of adsorption?
Marks:1View AnswerAnswer:
Chemisorption has a higher enthalpy of adsorption.
-
Q2
Name the method used for the refining of copper metal.
Marks:1View AnswerAnswer:
Electrolytic refining is used for the refining of copper metal.
-
Q3
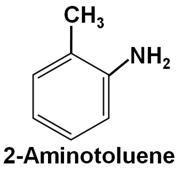
Write the structure of 2-aminotoluene.
Marks:1View AnswerAnswer:
The structure of 2-aminotoluene is as follows:
-
Q4
Which aerosol depletes the ozone layer?
Marks:1View AnswerAnswer:
Chlorofluoro compounds (freons), which are used as propellants in aerosols, deplete the ozone layer.
-
Q5
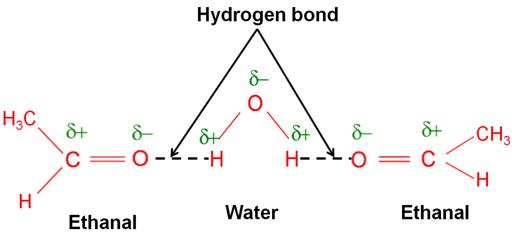
Ethanal is soluble in water. Why?
Marks:1View AnswerAnswer:
The solubility of ethanal in water is due to the formation of the hydrogen bonds between the polar carbonyl group and the water molecules.
-
Q6
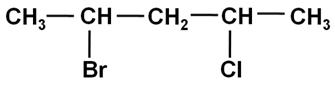
Write the IUPAC name of the following compound:
 Marks:1View Answer
Marks:1View AnswerAnswer:
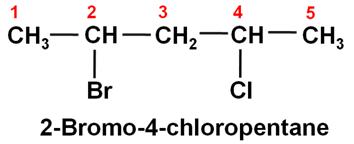
The IUPAC name of the given compound is:
-
Q7
Write the name of the linkage joining the two amino acids.
Marks:1View AnswerAnswer:
The two amino acid units are connected by the peptide bonds or the peptide linkage.
-
Q8
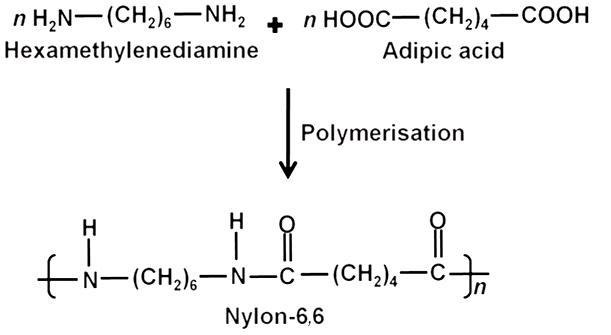
Give one example of a condensation polymer.
Marks:1View AnswerAnswer:
Nylon-6,6 is an condensation polymer, which is formed by the condensation polymerisation of the adipic acid and the hexamethylenediamine.
-
Q9
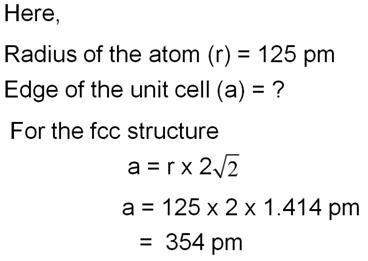
Aluminium crystallises in an fcc structure. The atomic radius of the metal is 125 pm. What is the length of the side of the unit cell of the metal?
Marks:2View AnswerAnswer:
-
Q10
The standard electrode potential (E°) for the Daniell cell is + 1.1 V. Calculate the
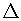 G° for the reaction
G° for the reaction

(1 F = 96500Cmol–1)Marks:2View AnswerAnswer:




