Chemistry:2013:CBSE:[ All India]:Set-I
To Access the full content, Please Purchase
-
Q1
Of physisorption or chemisorption, which has a higher enthalpy of adsorption?
Marks:1View AnswerAnswer:
Chemisorption has a higher enthalpy of adsorption.
-
Q2
Name the method used for the refining of copper metal.
Marks:1View AnswerAnswer:
Electrolytic refining is used for the refining of copper metal.
-
Q3
Name the two poisonous gases that can be prepared from the chlorine gas.
Marks:1View AnswerAnswer:
Mustard gas and phosgene gas are the poisonous gases that can be prepared from the chlorine gas.
-
Q4
Write the IUPAC name of the following compound:
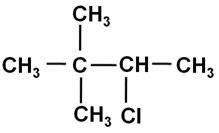 Marks:1View Answer
Marks:1View AnswerAnswer:
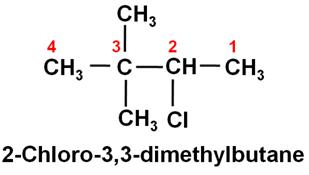
-
Q5
Rearrange the following compounds in the increasing order of their boiling points:
CH3–CHO, CH3–CH2–OH, CH3–CH2–CH3Marks:1View AnswerAnswer:
The increasing order of the boiling points is:
CH3–CH2–CH3 < CH3–CHO < CH3–CH2–OH -
Q6
Write the structure of N-methylethanamine.
Marks:1View AnswerAnswer:
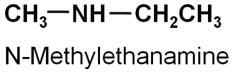
-
Q7
What are the products of the hydrolysis of sucrose?
Marks:1View AnswerAnswer:
On hydrolysis, sucrose gives an equimolar mixture of D-(+)-glucose and D-(–)-fructose.
-
Q8
 Marks:1View Answer
Marks:1View AnswerAnswer:
The repeating structural unit of the given polymer contains only one type of monomer unit, i.e. vinyl chloride, CH2=CH–Cl, therefore, it is a homopolymer.
-
Q9
Account for the following:
(i) Schottky defect lowers the density of the related solids.
(ii) Conductivity of silicon increases on doping it with phosphorus.Marks:2View AnswerAnswer:
(i) In the Schottky defect, the equal number of positive and negative ions is missing from the ionic crystal. As a result of this defect, the number of ions decreases. Due to this, the mass decreases whereas the volume remains the same. Hence, the density of the solid decreases.
(ii) When silicon is doped with P (group 15 element), the silicon atoms at some lattice sites are substituted by the atoms of P. Now, as these atoms have five valance electrons, after forming the four covalent bonds with the neighbouring silicon atoms, the fifth extra electron is free and gets delocalised. These delocalised electrons increase the conductivity of the silicon. -
Q10
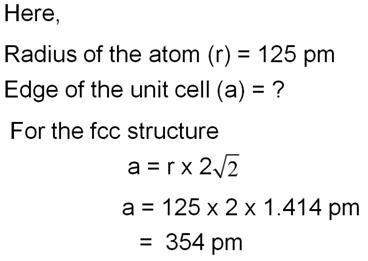
Aluminium crystallises in an fcc structure. The atomic radius of the metal is 125 pm. What is the length of the side of the unit cell of the metal?
Marks:2View AnswerAnswer: