Chemistry:2012:CBSE:[Delhi]: Set-I
To Access the full content, Please Purchase
-
Q1
What is meant by ‘doping’ in a semiconductor?
Marks:1View AnswerAnswer:
It is the process of increasing conductivity of the intrinsic semiconductors by adding suitable impurity.
-
Q2
What is the role of graphite in the electrometallurgy of aluminium?
Marks:1View AnswerAnswer:
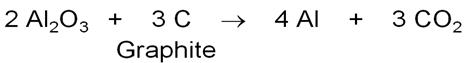
-
Q3
Give the IUPAC name of the following compound.
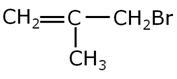
Marks:1View AnswerAnswer:
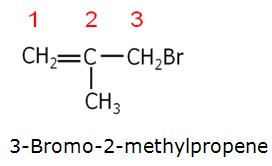
-
Q4
Define the term, ‘homopolymerisation’ giving an example.
Marks:1View AnswerAnswer:
The addition polymers which are formed by the polymerisation of one type of monomeric species are known as homopolymers and the process is known as homopolymerisation.
Example: Formation of polyethene (homopolymer of ethene)
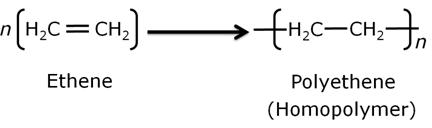
-
Q5
Arrange the following in the decreasing order of their basic strength in aqueous solutions:
CH3NH2, (CH3)2NH, (CH3)3N and NH3
Marks:1View AnswerAnswer:
Decreasing order of basic strength
(CH3)2NH > CH3NH2 > (CH3)3N > NH3 -
Q6
Arrange the following compounds in an increasing order of their reactivity in nucleophilic addition reactions: ethanal, propanal, propanone, butanone.
Marks:1View AnswerAnswer:
Incresing order of reactivity:
butanone < propanone < propanal < ethanal -
Q7
Which one is not likely to exist PCl4+ or PCl4-?
Marks:1View AnswerAnswer:
In PCl4- the oxidation state of phosphorus is +3 which is less stable. Therefore, PCl4- is less likely to exist.
-
Q8
Draw the structural formula of 2-methylpropan-2-ol molecule.
Marks:1View AnswerAnswer:
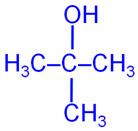
-
Q9
A 1.00 molal aqueous solution of trichloroacetic acid (CCl3COOH) is heated to its boiling point. The solution has the boiling point of 100.18°C. Determine the van’t Hoff factor for trichloroacetic acid. (Kb for water= 0.512 kg mol-1)
Marks:2View AnswerAnswer:

-
Q10
Define the following terms:
(i) Mole fraction
(ii) Isotonic solutions
(iii) van’t Hoff factor
(iv) Ideal solution
Marks:2View AnswerAnswer:
(i) Mole fraction:- It is the ratio of number of moles of one component (solute or solvent) to the total number of moles (solutes and solvent) present in a solution.

(ii) Isotonic solutions :- They are those solutions which have same osmotic pressure at same temperature.
(iii) van’t Hoff factor :- It is defined as the ratio of observed value of colligative property to the calculated value of colligative property. It is used to find out the extent of association or dissociation.
(iv) Ideal solution:-The solutions which obey Raoult’s law over the entire range of concentration is known as ideal solution.



