Chemistry:2012:CBSE:[All India]: Set-II
To Access the full content, Please Purchase
-
Q1
Which stoichiometric defect increases the density of a solid?
Marks:1View AnswerAnswer:
Interstitial defect
-
Q2
What is meant by ‘shape selective catalysis’?
Marks:1View AnswerAnswer:
The catalytic reaction that depends on pore structure of catalyst and shape and size of the reactants and products. E.g. Zeolites.
-
Q3
What is the role of collectors in Froth Flotation process?
Marks:1View AnswerAnswer:
Collectors enhances the non wetability of the mineral particles.
-
Q4
Which is stronger reducing agent, SbH3 or BiH3, and why?
Marks:1View AnswerAnswer:
Sb and Bi both belong to same group. BiH3 is stronger reducing agent because the tendency to liberate hydrogen increases as we move down the period.
-
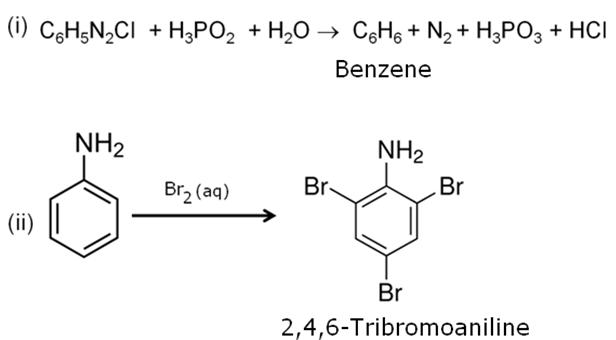
Q5
Write the structure of the product obtained when glucose is oxidized with nitric acid.
Marks:1View AnswerAnswer:
-
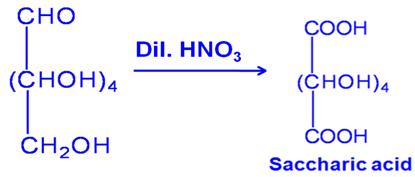
Q6
What happened when bromine attacks CH2=CH–CH2–CΞCH?
Marks:1View AnswerAnswer:
Bromine molecule attaches across C=C and the following compound is formed
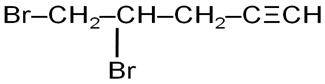
-
Q7
Write the IUPAC name of Ph–CH=CH–CHO.
Marks:1View AnswerAnswer:
IUPAC name is 3-Phenyl prop-2-en-1-al
-
Q8
Differentiate between disinfectants and antiseptics.
Marks:1View AnswerAnswer:
Antiseptics are applied to the living tissues such as wounds, cuts, ulcers, and diseased skin surfaces, while disinfectants are applied to inanimate objects such as floors, drainage system, instruments, etc.
-
Q9
Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol.
Marks:2View AnswerAnswer:
Alkenes undergo direct hydration in the presence of mineral acids which act as catalysts. The addition of water to the double bond takes place in accordance with Markonikoff's rule.
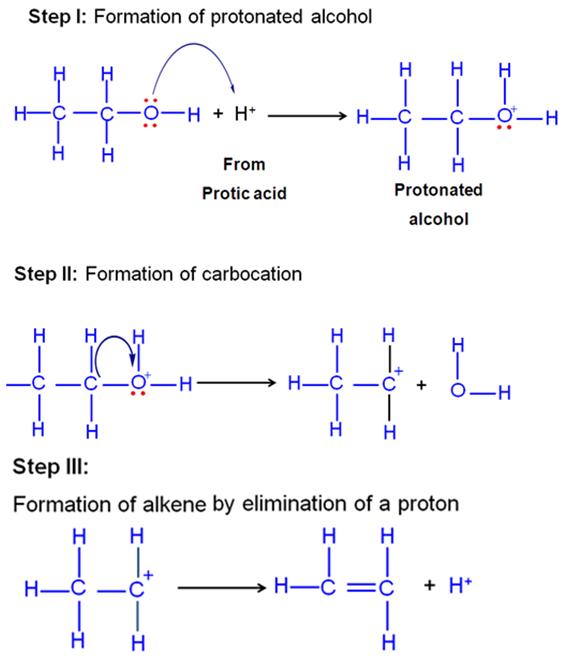
-
Q10
Complete the following chemical reaction equations:
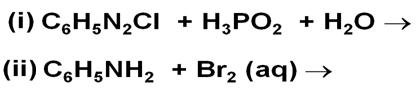 Marks:2View Answer
Marks:2View AnswerAnswer: