Chemistry:2012:CBSE:[All India]: Set-I
To Access the full content, Please Purchase
-
Q1
How may the conductivity of an intrinsic semiconductor be increased?
Marks:1View AnswerAnswer:
The conductivity of an intrinsic semiconductor can be increased by increasing the temperature.
-
Q2
Which is stronger reducing agent, SbH3 or BiH3, and why?
Marks:1View AnswerAnswer:
Sb and Bi both belong to same group. BiH3 is stronger reducing agent because the tendency to liberate hydrogen increases as we move down the period.
-
Q3
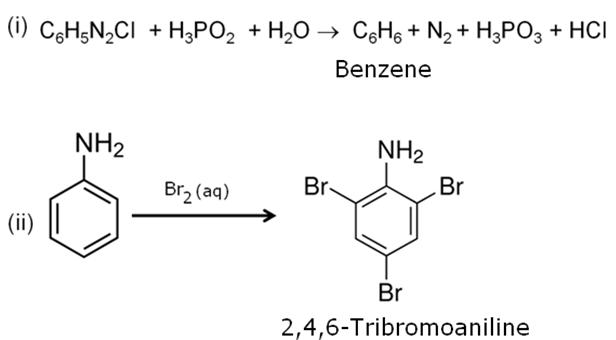
Write the structure of the product obtained when glucose is oxidized with nitric acid.
Marks:1View AnswerAnswer:
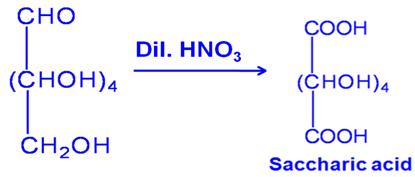
-
Q4
What happened when bromine attacks CH2=CH–CH2–CΞCH?
Marks:1View AnswerAnswer:
Bromine molecule attaches across C=C and the following compound is formed
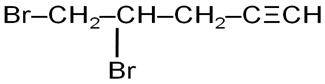
-
Q5
Define 'peptization'.
Marks:1View AnswerAnswer:
Peptization is the process of conversion of a precipitate into a colloidal sol by shaking it with the dispersion medium in the presence of an electrolyte. The electrolyte used in this reaction is known as a peptizing agent.
-
Q6
How is copper extracted from a low grade ore of it?
Marks:1View AnswerAnswer:
Copper is extracted by hydrometallurgy from low grade ores.
-
Q7
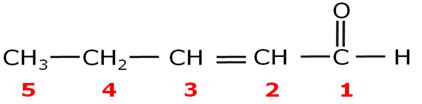
Write the IUPAC name of the following.
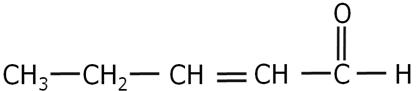 Marks:1View Answer
Marks:1View AnswerAnswer:
Pent-2-en-1-al
-
Q8
Differentiate between disinfectants and antiseptics.
Marks:1View AnswerAnswer:
Antiseptics are applied to the living tissues such as wounds, cuts, ulcers, and diseased skin surfaces, while disinfectants are applied to inanimate objects such as floors, drainage system, instruments, etc.
-
Q9
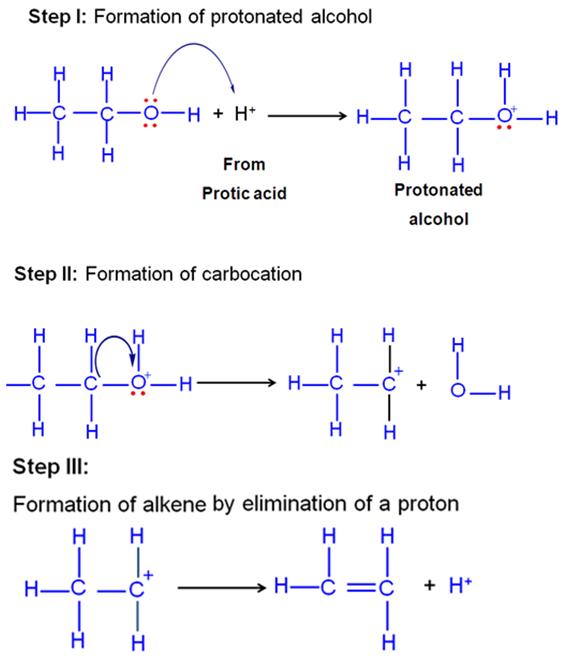
Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol.
Marks:2View AnswerAnswer:
Alkenes undergo direct hydration in the presence of mineral acids which act as catalysts. The addition of water to the double bond takes place in accordance with Markonikoff's rule.
-
Q10
Complete the following chemical reaction equations:
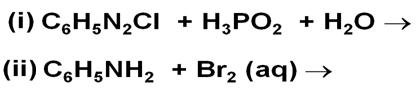 Marks:2View Answer
Marks:2View AnswerAnswer: