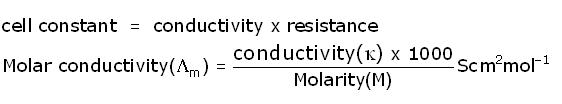Chemistry:2010:CBSE:[All India]:Set-II
To Access the full content, Please Purchase
-
Q1
What is meant by ‘reducing sugars’?
Marks:1View AnswerAnswer:
A sugar that contains an aldehyde group/s that can be easily oxidised to carboxylic acid/s by mild oxidising agent is known as reducing sugar.
-
Q2
Fluorine does not exhibit any positive oxidation state. Why?
Marks:1View AnswerAnswer:
Fluorine is the most electronegative element; therefore, it always shows -1 oxidation state and does not show any positive oxidation state.
-
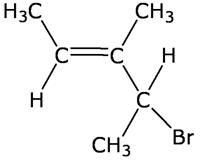
Q3
Give the IUPAC name of the following compound:
 Marks:1View Answer
Marks:1View AnswerAnswer:
4-Bromo-3-methyl-pent-2-ene
-
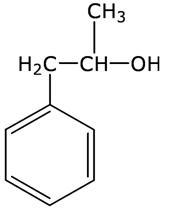
Q4
Write the structure of the molecule of a compound whose IUPAC name is 1-phenylpropan-2-ol
Marks:1View AnswerAnswer:
-
Q5
Nitrogen is relatively inert as compared to phosphorus. Why?
Marks:1View AnswerAnswer:
In nitrogen molecule, triple bond exists between two nitrogen atoms. In phosphorus molecule, single bond is present between atoms of phosphorus. Since bond dissociation energy is more for triple bond as compared to that of single bond, nitrogen is relatively inert as compared to phosphorus.
-
Q6
What type of semiconductor is obtained when silicon is doped with arsenic?
Marks:1View AnswerAnswer:
n-type semiconductor is obtained when silicon is doped with arsenic.
-
Q7
What are monosaccharides?
Marks:1View AnswerAnswer:
Monossacharides are simplest carbohydrates which cannot be hydrolysed to simpler molecules. They are represented by general formula (CH2O)n.
-
Q8
What is meant by copolymerisation?
Marks:1View AnswerAnswer:
The process of formation of polymer by addition polymerisation of two different monomeric species is called copolymerisation.
-
Q9
Describe the following:
(i) Tyndall effect
(ii) Shape selective catalysis
Marks:2View AnswerAnswer:
(i) Tyndall effect: When a beam of light is passed through a colloidal system, the colloidal particles scatter the light and the path of beam becomes illuminated. This scattering of light by colloidal particles is known as Tyndall effect.
(ii) Shape selective catalysis: The catalytic reaction that depends upon the pore structure of the catalyst and the size of reactant and product is called shape selective catalysis.
-
Q10
Express the relation among the cell constant, the resistance of the solution in the cell and the conductivity of the solution. How is the conductivity of a solution related to its molar conductivity?
Marks:2View AnswerAnswer: