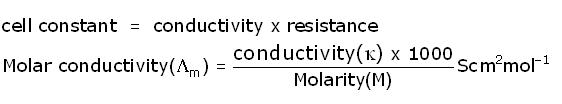Chemistry:2010:CBSE:[All India]:Set-I
To Access the full content, Please Purchase
-
Q1
What type of interactions hold the molecules together in a polar molecular solid?
Marks:1View AnswerAnswer:
In a polar molecular solid the molecules are held together by dipole-dipole forces.
-
Q2
What is Tollen’s reagent? Write one usefulness of this reagent.
Marks:1View AnswerAnswer:
Tollen’s reagent is ammoniacal solution of AgNO3. It is used to distinguish between aldehyde and ketone groups.
-
Q3
What is meant by ‘reducing sugars’?
Marks:1View AnswerAnswer:
A sugar that contains an aldehyde group/s that can be easily oxidised to carboxylic acid/s by mild oxidising agent is known as reducing sugar.
-
Q4
Name the monomer of starch.
Marks:1View AnswerAnswer:
Monomer of starch is alpha D-Glucose.
-
Q5
What is meant by ‘limiting molar conductivity’?
Marks:1View AnswerAnswer:
Molar conductivity is the conducting potential of all ions produced by dissolving one mole of an electrolyte in V cc of the solution. Molar conductivity at infinite dilution is called limiting molar conductivity.
-
Q6
Fluorine does not exhibit any positive oxidation state. Why?
Marks:1View AnswerAnswer:
Fluorine is the most electronegative element; therefore, it always shows -1 oxidation state and does not show any positive oxidation state.
-
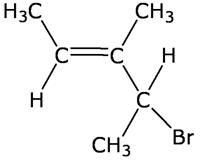
Q7
Give the IUPAC name of the following compound:
 Marks:1View Answer
Marks:1View AnswerAnswer:
4-Bromo-3-methyl-pent-2-ene
-
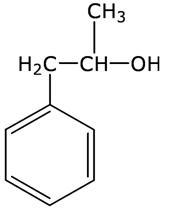
Q8
Write the structure of the molecule of a compound whose IUPAC name is 1-phenylpropan-2-ol
Marks:1View AnswerAnswer:
-
Q9
Describe the following:
(i) Tyndall effect
(ii) Shape selective catalysis
Marks:2View AnswerAnswer:
(i) Tyndall effect: When a beam of light is passed through a colloidal system, the colloidal particles scatter the light and the path of beam becomes illuminated. This scattering of light by colloidal particles is known as Tyndall effect.
(ii) Shape selective catalysis: The catalytic reaction that depends upon the pore structure of the catalyst and the size of reactant and product is called shape selective catalysis.
-
Q10
Express the relation among the cell constant, the resistance of the solution in the cell and the conductivity of the solution. How is the conductivity of a solution related to its molar conductivity?
Marks:2View AnswerAnswer: