Chemistry:2005:CBSE:[Delhi]:Set-II
To Access the full content, Please Purchase
-
Q1
Express the relation between the half-life period of a reactant and its initial concentration if the reaction involved is of second order . Marks:1View AnswerAnswer:
The relation between the half-life period of a reactant and its initial concentration of second order reaction is:
Half life
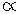 1/ Initial concentration of reactant
1/ Initial concentration of reactant
-
Q2
State the formula relating pressure of a gas with its mole fraction in a liquid solution in contact with it. Marks:1View AnswerAnswer:
This relation is given by Henry:- p = KH x XA
Where p = Vapour pressure of the gas
KH = Henry’s constant
XA = Mole fraction of gas in liquid. -
Q3
What is the maximum possible coordination number of an atom in an hcp crystal structure of an element? Marks:1View AnswerAnswer:
The maximum possible coordination number of an atom in an hcp crystal structure of an element is 12. -
Q4
Why primary amines have higher boiling than tertiary amines. Marks:1View AnswerAnswer:
Primary amines have higher boiling point than tertiary amines because primary amines form intermolecular hydrogen bonding but tertiary amines do not form intermolecular hydrogen bonding due to absence of hydrogen atom. -
Q5
How are formalin and trioxane related to methanal? Marks:1View AnswerAnswer:
Formalin:- It is a 40% aqueous solution of methanal.
Trioxane:- It is a trimer of methanal and it is also known as metaformaldehyde. -
Q6
On the basis of Heisenberg Uncertainty Principle show that the electron (mass=9 x 10 -31 kg) cannot exist within an atomic nucleus of radius 10 -15 m. out of syllabus Marks:2View AnswerAnswer:
This question is not in 2007-2008 syllabus. -
Q7
Show that the Heisenberg Uncertainty Principle is of negligible significance for an object of 10 -6 kg mass.
h/ 4 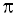 = 0.528
= 0.528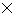 10-34 kg m2s-1
10-34 kg m2s-1 Out of syllabus
Marks:2View AnswerAnswer:
This question is not in 2007-2008 syllabus. -
Q8
Write one chemical reaction each to show that
(a) Tin (II) chloride is a reducing agent.
(b) Chlorine gas can be obtained from bleaching powder.Marks:2View AnswerAnswer:
a) Tin (II) chloride is a reducing agent:
2FeCl3 + SnCl2 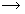
SnCl4 + 2FeCl2
(b) Chlorine gas can be obtained from bleaching powder:
Ca(OCl)Cl + 4HCl 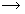
CaCl2 + 2H2O + 2Cl2 -
Q9
Describe the steps involved in the preparation of either potassium dichromate from sodium chromate or potassium permanganate from manganese dioxide. Marks:2View AnswerAnswer:
Preparation of Potassium dichromate from sodium chromate:
-In this preparation three steps are involved and these steps are:-
(i) Preparation of sodium chromate :- Finely powdered chromite ore is mixed with soda ash and quicklime. The mixture is then roasted in a reverberatory furnace in the presence of air. Yellow mass due to the formation of sodium chromate is obtained. 4 Fe2Cr2O4 + O2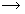 2 Fe2O3 + 4 Cr2O3 4 Cr2O3 + 8 Na2CO3 + 6 O2
2 Fe2O3 + 4 Cr2O3 4 Cr2O3 + 8 Na2CO3 + 6 O2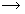 8 Na2Cr2O4 + 8 CO2
8 Na2Cr2O4 + 8 CO24 Fe2Cr2O4+ 7 O2 + 8Na2CO3 2Fe2O3+8 CO2 + 8 Na2Cr2O4
(ii) Conversion of chromate into dichromate:-
The yellow solution of Chromate is filtered and treated with concentrated sulphuric acid to give a solution from which orange sodium dichromate (Na2Cr2O7.2H2O) can be crystalized. 2 Na2Cr2O4 + H2SO4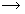 Na2Cr2O7 + Na2SO4 + H2O
Na2Cr2O7 + Na2SO4 + H2OSodium Chromate Sodium dichromate
(iii) Conversion of sodium dichromate to potassium dichromate:- Hot concentrated solution of sodium dichromate is treated with a calculated amount of potassium chloride, when potassium dichromate being less soluble crystallizes out on cooling.
Na2Cr2O7 + 2KCl
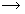 K2Cr2O7 + 2NaCl
K2Cr2O7 + 2NaCl
-
Q10
What are enantiomers and diastereomers? Differentiate between chiral and achiral molecules. Marks:2View AnswerAnswer:
Enantiomers:- Stereoisomers which are non-superimposable mirror images to each other are known as enantiomers or enantiomorphs. Diastereomers:- The stereoisomers which are optically active isomers but not the mirror images of each other are called diastereoisomers.
An object which is not superimposable on its mirror image is said to be chiral whereas the object which are superimposable on its mirror image is said to be achiral.



