Chemistry:2004:CBSE:[Delhi]:Set-III
To Access the full content, Please Purchase
-
Q1
Write the IUPAC name of the following :- (CH3)2C=CHCOCH3 Marks:1View AnswerAnswer:
The IUPAC name of (CH3)2C=CHCOCH3 is 4-methylpent-3-en-2-one. -
Q2
Mention two properties of acetonitrile because of which it acts as a good solvent. Marks:1View AnswerAnswer:
Acetonitrile is used as a solvent for performing many organic reactions because:– (1) It has high polarity, so, it is capable of dissolving a variety of solvents. (2) It is soluble in water. -
Q3
What makes alkali metal halides sometimes coloured, which are otherwise colourless? Marks:1View AnswerAnswer:
Due to F centres or electron trapped in anionic vacancies, alkali metal halides are sometimes coloured otherwise they are colourless. -
Q4
State Henry's law about the solubility of a gas in a liquid . Marks:1View AnswerAnswer:
According to Henry's law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
p = KH X -
Q5
Define the order of a reaction . Marks:1View AnswerAnswer:
The order of reaction is the sum of the concentration power terms in an experimentally determined rate of a reaction. -
Q6
Write the cell reactions which occur in lead storage battery (i) When the battery is in use. (ii) When the battery is on charging. Marks:2View AnswerAnswer:
The cell reaction occurring in lead storage battery (i) When the battery is in use:– At anode - PbSO4(s) + 2e- → Pb(s) + SO42-(aq) At cathode – PbO2(s) + SO42-(aq) + 4H+(aq) + 2e- → PbSO4(s) + 2H2O(l)
(ii) When the battery is on charging –The reaction is reversed on charging. So, PbSO4(s) on anode and cathode is converted into Pb and PbO2 respectively. -
Q7
Give chemical reaction in support of each of the following statements: (i) The +1 oxidation state gets stabilised progressively from Ga to Ti in Group 13
(ii) All the bonds in PCl5 are not equivalent.Marks:2View AnswerAnswer:
(i) This question is not in 2007-2008 syllabus. (ii) PCl5,when reacts with water,first POCl3 and then phosphoric acid are formed. PCl5 + H2O → POCl3 + 2HCl POCl3 + 3H2O → H3PO4 + 3HCl As two axial P-Cl bonds are repelled by three bond pairs ,but three equatorial bonds are repelled by 2 bond pairs. So, axial bonds are longer than equatorial bonds. -
Q8
Explain the following terms:
(a) Asymmetric molecule
(b) R and S notationsMarks:2View AnswerAnswer:
(a) Asymmetric molecule - When all the four atoms or groups attached to the carbon atom are different, then the molecule is called an asymmetric molecule. e.g. C F Cl Br I (all the 4 halogens are attached to C atom) .The carbon atom is called chiral carbon atom. An asymmetric molecule is known as enantiomers with non- super imposable mirror images. (b) This question is not in 2007-2008 syllabus. -
Q9
Write the names of the reagents and equations in the conversion of :-
(a) Phenol to salicylic aldehyde
(b) anisole to p-methoxyacetophenoneMarks:2View AnswerAnswer:
(a) Phenol to salicylic aldehyde:–
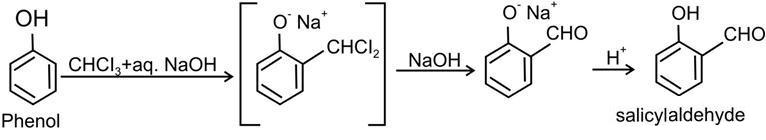
The above reaction is called Reimer-Tiemann reaction. (b) Anisole to p-methoxyacetophenone:- 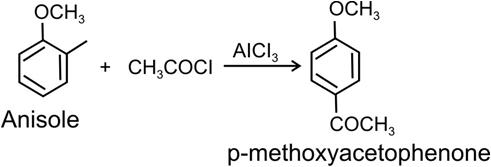
-
Q10
Write the modes of free radical polymerization of an alkene. Marks:2View AnswerAnswer:
Polymerization of alkene takes place through radicals which are generated by an initiator. An initiator is a molecule that decompose to provide radicals easily. e.g. t-butyl peroxide is used as initiator because it decomposes under mild conditions to form t-butoxide radical.
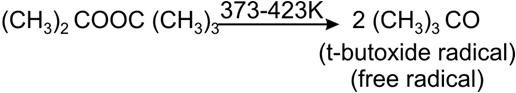
This free radical adds to a monomer molecule to form a free radical of larger size. These repeated additions form polymers. 



