Chemistry:2004:CBSE:[Dehli]:Set-I
To Access the full content, Please Purchase
-
Q1
Name a substance which on addition to AgCl causes cation vacancy in it. Marks:1View AnswerAnswer:
When CdCl2 or SrCl2 is added to AgCl , it gives a solid solution where divalent Cd2+ or Sr2+ occupies the Ag+ site and results in cation vacancies which are equal in number to that of the divalent ions. -
Q2
Mention a large scale use of the phenomenon called ‘reverse osmosis’. Marks:1View AnswerAnswer:
In large scale, the reverse osmosis is used for the desalination of sea water. -
Q3
Give an example of a pseudo first order reaction. Marks:1View AnswerAnswer:
The hydrolysis of cane sugar in presence of some mineral acids like HCl or H2SO4 is an example of a pseudo first order reaction.
C12H22O11 + H2O →C6H12O6 + C6H12O6 Glucose Fructose -
Q4
Write the IUPAC name of the following :- (CH3)2C=CHCOCH3 Marks:1View AnswerAnswer:
The IUPAC name of (CH3)2C=CHCOCH3 is 4-methylpent-3-en-2-one. -
Q5
Mention two properties of acetonitrile because of which it acts as a good solvent. Marks:1View AnswerAnswer:
Acetonitrile is used as a solvent for performing many organic reactions because:– (1) It has high polarity, so, it is capable of dissolving a variety of solvents. (2) It is soluble in water. -
Q6
Draw a diagram showing the formation of bonding and antibonding molecular orbital by the LCAO in homonuclear hydrogen molecule. out of syllabus Marks:2View AnswerAnswer:
The
diagram showing the formation of bonding and antibonding molecular orbitals by the Linear Combination of Atomic Orbitals (LCAO) in homonuclear hydrogen molecule is shown below:- 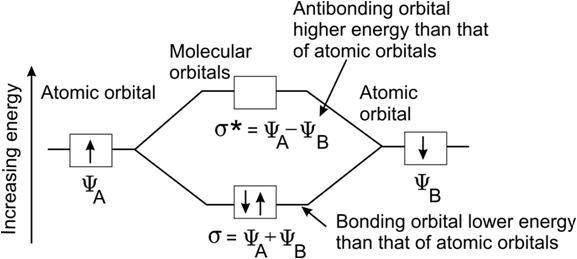
-
Q7
Taking a specific example show that ∆S total is a criterion for spontaneity of a change.out of syllabus Marks:2View AnswerAnswer:
Out of syllabus -
Q8
Write the cell reactions which occur in lead storage battery (i) When the battery is in use. (ii) When the battery is on charging. Marks:2View AnswerAnswer:
The cell reaction occurring in lead storage battery (i) When the battery is in use:– At anode - PbSO4(s) + 2e- → Pb(s) + SO42-(aq) At cathode – PbO2(s) + SO42-(aq) + 4H+(aq) + 2e- → PbSO4(s) + 2H2O(l)
(ii) When the battery is on charging –The reaction is reversed on charging. So, PbSO4(s) on anode and cathode is converted into Pb and PbO2 respectively. -
Q9
Give chemical reaction in support of each of the following statements: (i) The +1 oxidation state gets stabilised progressively from Ga to Ti in Group 13
(ii) All the bonds in PCl5 are not equivalent.Marks:2View AnswerAnswer:
(i) This question is not in 2007-2008 syllabus. (ii) PCl5,when reacts with water,first POCl3 and then phosphoric acid are formed. PCl5 + H2O → POCl3 + 2HCl POCl3 + 3H2O → H3PO4 + 3HCl As two axial P-Cl bonds are repelled by three bond pairs ,but three equatorial bonds are repelled by 2 bond pairs. So, axial bonds are longer than equatorial bonds. -
Q10
Explain the following terms:
(a) Asymmetric molecule
(b) R and S notationsMarks:2View AnswerAnswer:
(a) Asymmetric molecule - When all the four atoms or groups attached to the carbon atom are different, then the molecule is called an asymmetric molecule. e.g. C F Cl Br I (all the 4 halogens are attached to C atom) .The carbon atom is called chiral carbon atom. An asymmetric molecule is known as enantiomers with non- super imposable mirror images. (b) This question is not in 2007-2008 syllabus.



